252 serum samples of sixty-three patients with MF/SS were collected and evaluated by immunoassay. Sixty-three (100%), 48 (76%), and 41 (65%) patients had baseline, post-radiation, and last follow-up samples, respectively. The median follow-up for this analysis was 12 months. The median age was 63 years (range: 38-87). The patient population consisted of 52 (83%) MF and 11 (17%) SS patients. The baseline concentrations of sPD-L1, sCD30, and sTARC biomarkers are significantly higher in MF/SS patients than in healthy individuals. Low baseline sPD-L1 and sCD30 levels were associated with higher response rates (P<0.05). At the end of treatment. The level of sPD-L1, sCD30, and sTARC concentrations was associated with the clinical outcome (Figure 1). In a Cox proportional hazard model, CLIC-risk group, ECOG score, baseline WBC count, CRP level, posttherapy level of sPD-L1, sCD30, sTARC, sCTLA-4, sIL-2R: soluble interleukin 2 receptor, interleukin 6, and clinical response to radiotherapy were included. In the multivariate analysis, the posttherapy sPD-L1 and sCD30 levels were associated with progression-free survival (PFS). At the same time point, the sTARC level and ECOG score proved to be significant determinants of overall survival (OS).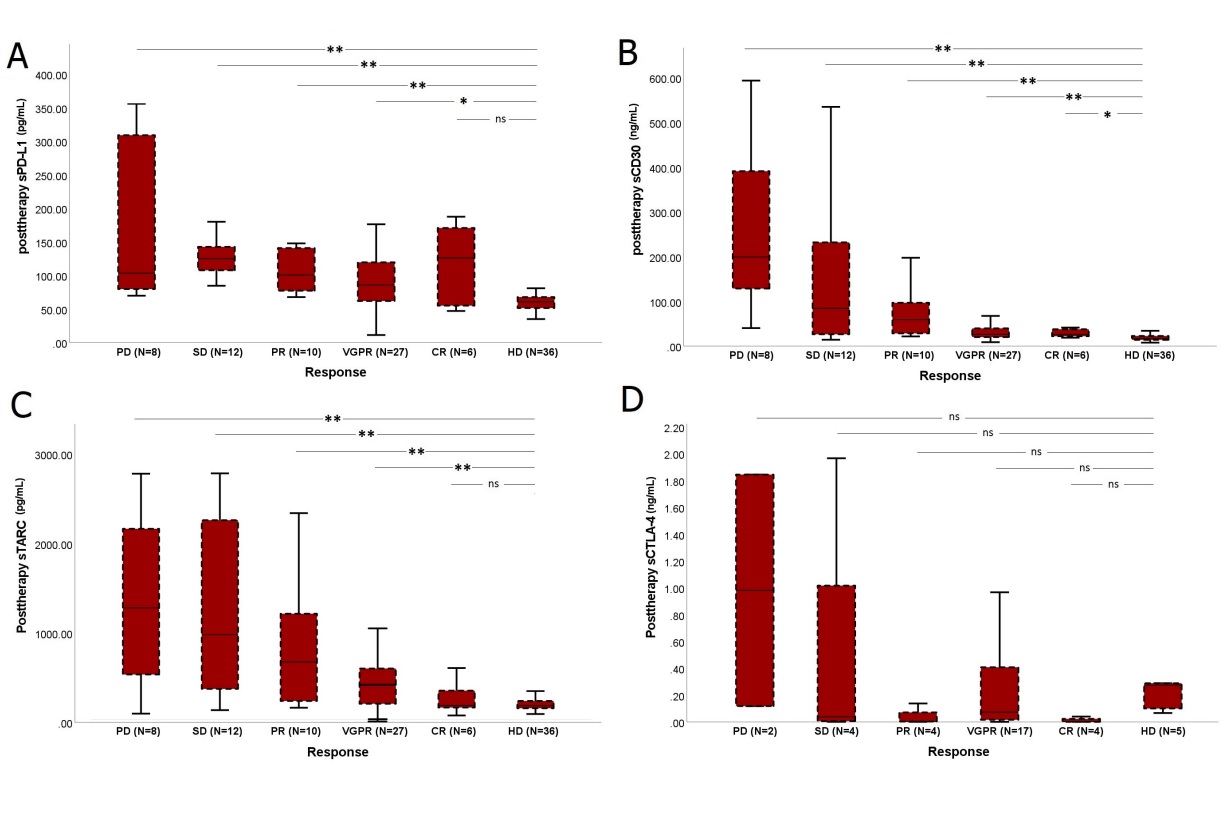
Abbreviations: complete remission (CR), very good partial response (VGPR), partial remission, stable disease (SD), and disease progression (PD).
Figure 1: The level of soluble biomarkers’ concentrations in healthy individuals and according to posttherapy outcome in treated patients (N=63).