In this presentation we will describe the SAMIRA study for medical equipment focused on radiotherapy, a tender with the European Comission under the SAMIRA action plan. SAMIRA foresees generating high quality evidence and developing evidence-based guidance and practical tools to improve the quality and safety of medical applications of ionising radiation and to support the implementation of Council Directive 2013/59/Euratom (Basic Safety Standards, BSS). From other SAMIRA tenders, projects such as SIMPLERAD, in which EFOMP also participates, or QUADRANT, in which ESTRO participates jointly with ESR and EANM, are also being carried out.
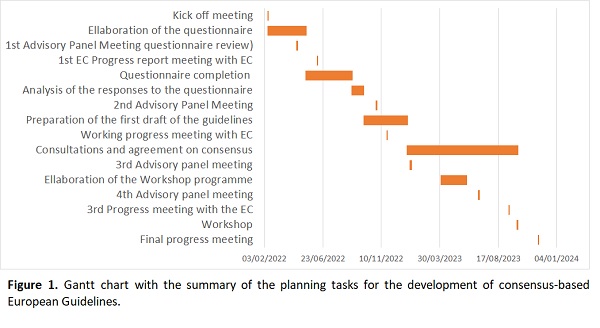
The process for developing consensus-based guidelines is described in the task summary in the Gantt chart (Fig. 1). The main tasks are the elaboration of a questionnaire sent to the national radiation protection authorities and medical physics organizations in each of the 30 countries (EU-27 and UK, Switzerland, and Norway), the analysis of the responses, the elaboration of draft guidelines based on the responses to the questionnaire, the search for a consensus for these guidelines and finally the holding of a workshop. Each task is mainly performed by the successful bidders (EFOMP and Nuc Advisor), evaluated by the European Commission, and reviewed by the advisory panel.
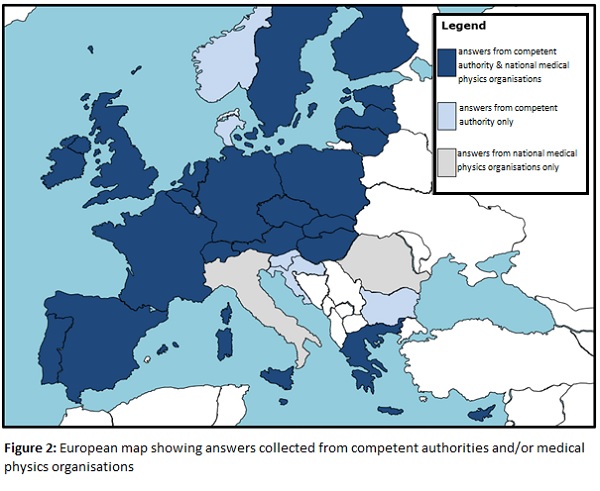
It started with the elaboration of a survey that can be filled out via the web that was sent to all national radiation protection authorities and national medical physics organizations. Participation has been very high, with at least one response from the 30 countries surveyed, and 22 countries with responses from both institutions, as can be seen in the map in Figure 2. The survey contains 90 questions for medical physics organizations and 76 for national competent authorities covering the different aspects of BSS compliance related to nuclear medicine, diagnostic radiology and radiotherapy equipment.
Based on the responses to the questionnaire, a report was prepared. Twenty-eight takeaway messages were identified in the three areas, of which ten were relevant to radiotherapy. This report, which includes the takeaway messages and the outline for the guidelines, has been reviewed by the European Commission and the Advisory Panel.
The vast majority of radiotherapy equipment installed in the EU complies with the requirement of article 60 (3.b) of the BSS and the use of record & verify systems (RVS) across Europe, with national mandatory regulatory use in some countries tend to confirm the level of compliance as evaluated by competent authorities in the survey. Key treatment parameters are usually not defined within current national regulations, leaving professional associations, equipment manufacturers and radiotherapy departments to define their own strategies for such data recording and verification.
RVS became the standard to ensure the verification of key treatment parameters in radiation therapy departments and some countries pushed for their adoption within their national regulation. Yet, some open challenges still need to be addressed. For example, RVS do not include information about doses for imaging. IGRT occupies an increasingly important place in patient positioning and the doses related to these images should be included in the final dose report. It is necessary to establish Dose Reference Levels for simulation imaging (CT) and positioning in radiotherapy (CBCT, portal imaging…) at European level, as was done for radiodiagnosis.
The first draft of the guidelines was delivered on January 10 to the European Commission and the Advisory Panel and be discussed on January 17 and 19. From then on, the process of consensus building will begin. In April, work begins on the program for the workshop, which will be held in September 2023 and where the guides developed will be disseminated.
This study has received funding from the European Commission under Service Contract N° ENER/LUX/2021/OP/0014.
The information and views set out in this presentation are those of the authors and do not necessarily reflect the official opinion of the European Commission. The Commission does not guarantee the accuracy of the data included in this study. Neither the Commission nor any person acting on the Commission’s behalf may be held responsible for the use which may be made of the information contained therein