Purpose: Head and Neck Squamous Cell Carcinoma (HNSCC) is the sixth most common cancer worldwide, resulting in more than 450.000 deaths a year. Half of the patients with advanced HNSCC die within five years. Most treatments aim to kill cancer cells, but this is often insufficient to cure advanced HNSCC. Besides cancer cells, also cancer-associated fibroblasts (CAFs) make up a large part of the tumour. Their presence, however, is often ignored. Currently, the majority of HNSCC patients will be treated with radiotherapy. Despite the abundance of CAFs, the effect of radiotherapy on their function in HNSCC is largely unknown. To increase the success rate of treatment strategies in HNSCC, more in-depth knowledge regarding CAFs and the effect of radiotherapy on their role is essential.
Materials and methods: CAFs were isolated from HNSCC patients and exposed to ionizing radiation ex vivo. The effect of different doses of radiation on CAFs was determined by microscopic analyses of cell growth, cell size, DNA damage and quantification of senescence.
Results: Our data shows that human HNSCC patient-derived CAFs are widely affected by radiotherapy; they dose-dependently decrease cell growth (Fig1A), increase their cell size (Fig.B,C) and have permanent DNA damage as measured by the presence of 53BP1 (Fig1D,E,F). As these effects are often associated with senescence, we established -and confirmed- radiation-induced senescence by staining the irradiated CAFs for β-galactosidase (Fig1G,H), the most commonly used marker to identify cellular senescence.
Conclusion: Our data show that radiotherapy modulates the phenotype of HNSCC patient-derived CAFs. Radiation of CAFs affects their morphology and induces senescence. Current research focuses on the effect of radiotherapy on the function of CAFs in vivo and their effect to modulate the anti-tumour immune response.
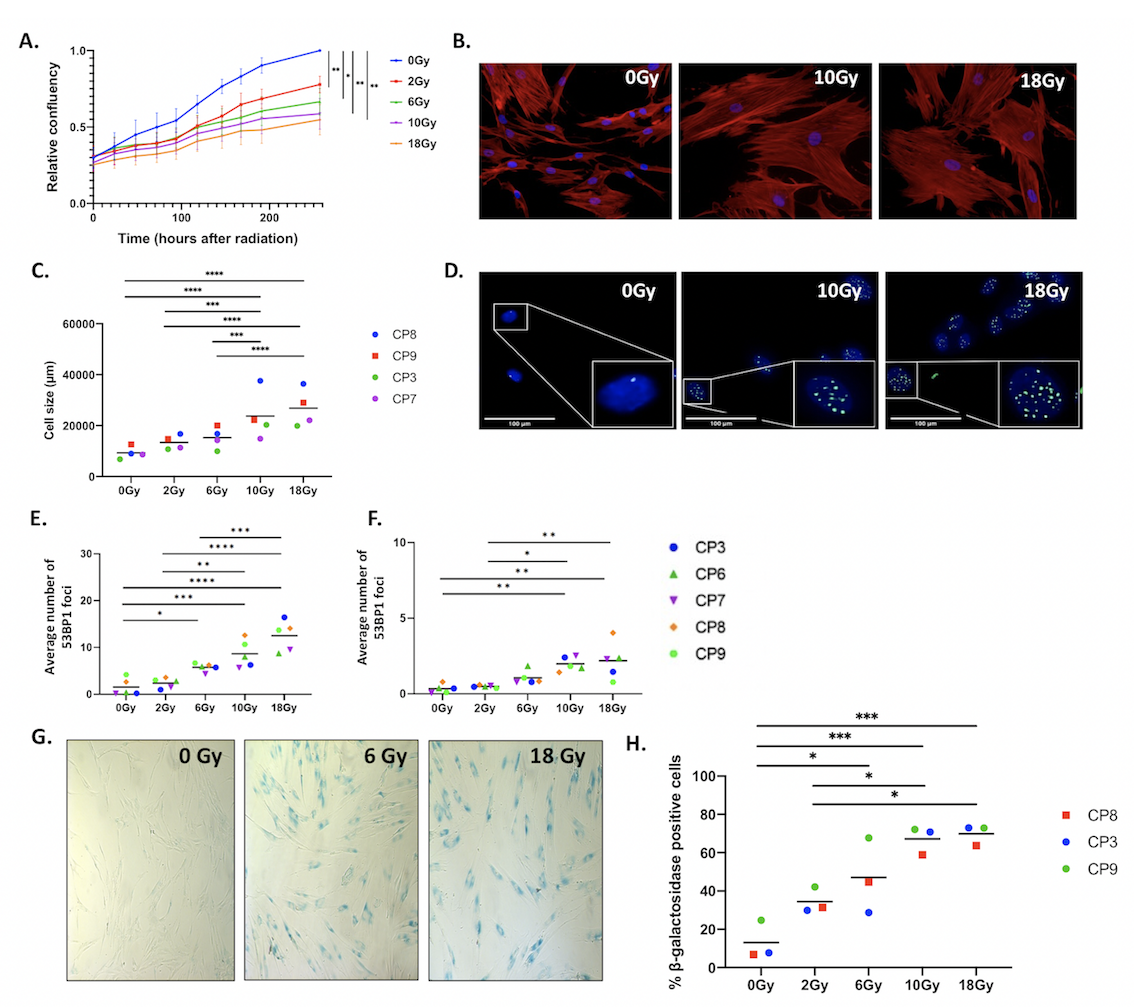
Figure 1 Radiotherapy affects HNSCC derived CAFs A) Primary CAFs isolated from 5 HNSCC patients were cultured and subjected to irradiation (0, 2, 6, 10 or 18Gy). Cell growth was monitored with the Incucyte Live-Cell Analysis System. B) Representative fluorescent images of actin (red) and Hoechst (nucleus, blue) of HNSCC patient-derived CAFs, 11 days after irradiation. C) Average cell of 4 HNSCC patient-derived CAFs (CP3, CP7, CP8 and CP9), 11 days after irradiation. D) Representative fluorescent images of 53BP1 (green) and Hoechst (nucleus, blue), 24hrs after irradiation. E) Average number of CP3, CP6, CP7, CP8 and CP9 53BP1 foci 24hr, or F) 6 days after irradiation. G) Representative brightfield image of β-galactosidase staining of HNSCC patient-derived CAFs 11 days after irradiation. H) Average percentage of CP3, CP8 and CP9 b-galactosidase positive cells, 11 days after irradiation.