Radiobiology Best Paper: Hypoxic tumour cells drive tumour relapse after radiotherapy as revealed by a novel tracing tool
Apostolos Menegakis,
The Netherlands
OC-0511
Abstract
Hypoxic tumour cells drive tumour relapse after radiotherapy as revealed by a novel tracing tool
Authors: Apostolos Menegakis1,2, Claire Vennin3, Jonathan Ient4, Rob Klompmaker2, Lenno Krenning2, Anoek Friskes2, Mila ilic2, Rolf Harkes5, Arjan Groot4, Jacco van Rheenen3, Marc Vooijs4, Rene Medema2
1Netherlands Cancer Institute (NKI), Radiation Oncology, Amsterdam, The Netherlands; 2Netherlands Cancer Institute (NKI), Cell Biology, Amsterdam, The Netherlands; 3Netherlands Cancer Institute (NKI), Molecular Pathology, Amsterdam, The Netherlands; 4GROW School for Oncology and Developmental Biology, Maastricht University Medical Centre, Radiation Oncology (Maastro), Maastricht, The Netherlands; 5Netherlands Cancer Institute (NKI), Bioimaging facility, Amsterdam, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Tumour hypoxia imposes a main obstacle to the efficacy of anti-cancer therapy, especially for radiotherapy and represents an important negative prognostic factor for treatment outcome and tumour progression of most solid tumours. Understanding the cellular dynamics of individual hypoxic cells prior, during and post-treatment will be of critical importance to understand their role in tumour progression and relapse.
Material and Methods
Here, we use H1299 lung adenocarcinoma cells expressing a novel lineage-tracing reporter of hypoxic cells to study the fate of this population after irradiation in tumor spheroids and in xenografts by means of multiphoton microscopy, flow cytometry analysis and immunofluorescence. Lineage tracing relies on the expression of a HIF1a-CreERT2-UnaG reporter (H1299-UnaG cells), which upon treatment with tamoxifen drives the sustained expression of UnaG in HIF1a-expressing cells.
Results
We first confirmed in 2D cultures the ability of the reporter to specifically label cells in hypoxia. In 3D cultures of H1299-UnaG spheroids, UnaG-positive cells are located in the central part of the spheroids, and do not proliferate in untreated spheroids. Irradiation of H1299-UnaG spheroids leads to a significant enrichment of UnaG-positive cells, indicating that the hypoxic cells are the main surviving population that drives spheroid regrowth. Upon IR they retain the integrity of the inner spheroid core while increased cell death is observed in the UnaG-negative cells. The surviving UnaG-positive cells despite being reoxygenated remain arrested for a prolonged period before they start to proliferate and give rise to regrowing spheroids.
Lastly, we generated H1299-UnaG xenograft tumors and found that UnaG-positive-cells coincide with pimonidazole-positive tumour areas and show absence of active cell cycle marker Ki-67 in untreated tumours. Upon IR, regrowing tumours show significantly higher percentage of UnaG-positive cells which are almost exclusively Ki67-positive compared to untreated controls revealed with cell-by-cell analysis of whole tumour cross-sections.
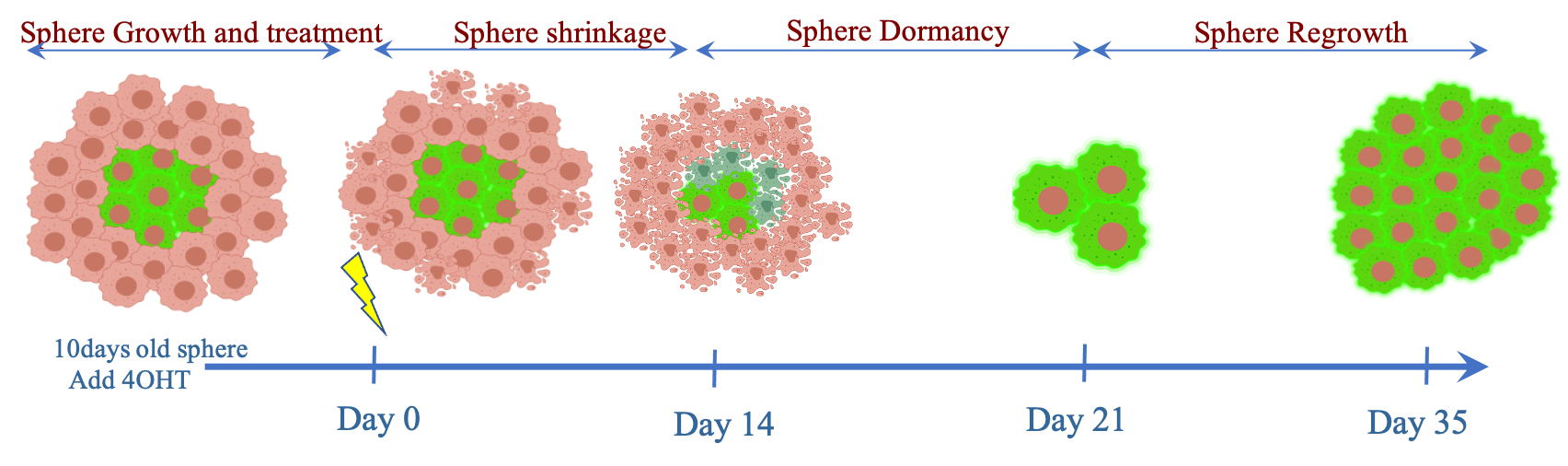
Conclusion
Collectively, our data demonstrate the feasibility to track individual tumour cells that were hypoxic at the time of irradiation and provide proof that the hypoxic tumour cells drive tumour relapse after irradiation.