Image-guided Stereotactic Body Radiotherapy for intermediate and high-risk endometrial carcinoma
Maria Ines Antunes,
Portugal
PD-0811
Abstract
Image-guided Stereotactic Body Radiotherapy for intermediate and high-risk endometrial carcinoma
Authors: Maria Ines Antunes1, Sandra Vieira1, Ana Soares1, Ariana Rocha1, Elda Freitas1, Joep Stroom1, Carlo Greco1
1Champalimaud Foundation, Radiotherapy, Lisbon, Portugal
Show Affiliations
Hide Affiliations
Purpose or Objective
To evaluate the feasibility of adjuvant image-guided Stereotactic Body Radiotherapy to the vaginal cuff (VC-SBRT) for intermediate and high-risk endometrial carcinoma (EC).
Material and Methods
Between February 2018 and November 2020, stage I to III EC patients who underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy with pelvic +/- paraaortic nodal sampling were treated in an institutional review board-approved study. The aim of the study was to reproduce adjuvant High Dose Rate (HDR) brachytherapy dose distributions with VC-SBRT.
Patients were submitted to SBRT +/- External Beam RT (EBRT) according to risk classification. Those with lower risk of relapse (Group 1) underwent VC-SBRT alone (21Gy in 3 fractions), whereas higher risk patients (Group 2) were treated with EBRT (46Gy/23 fractions) plus VC-SBRT (15Gy/2 fractions).
For both groups the vaginal cuff was treated to 100% of prescribed dose at a depth of 5 mm from the mucosa surface (vc) and to 150% to the mucosa surface (vcm). Both Pelvic EBRT and SBRT boost were treated using VMAT. The PTV boost was obtained from the CTV using an expansion margin of 3mm. Boost patient setup is shown in figure 1. Dose volume constraints for organs at risk (OARs) were defined according to the Gyn GEC ESTRO Recommendations.
Clinical evaluation was performed within 2 weeks after completion of SBRT, at 3 and 6 months, then every 6 months until the end of year 3, then annually. Toxicity evaluation was recorded according to Common Toxicity Criteria (version 2.0) (CTCAE) scores.
Estimated overall survival (OS) and disease-free survival (DFS) were calculated using Kaplan Meyer survival curves.
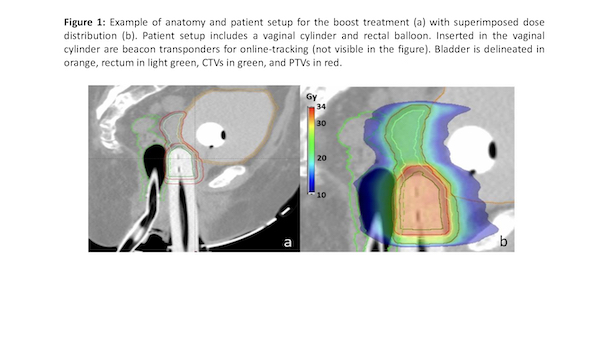
Results
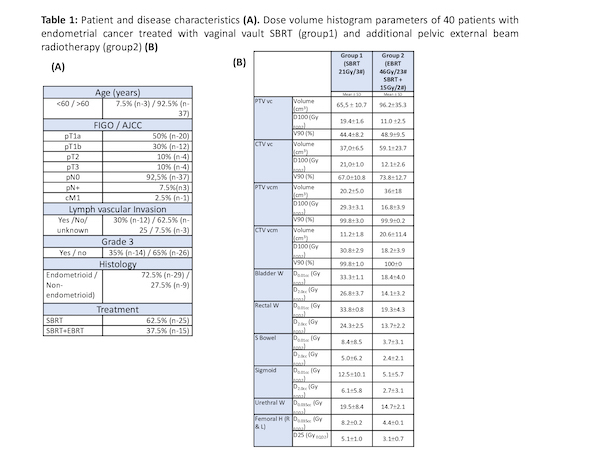
Forty patients (median age of 68 years) were accrued in the study. Patient and disease characteristics are summarized in table 1 (A). PTV coverage and dose to the OAR are presented in table 1 (B).
OS for the entire cohort is 92.5%; 100% for more prognostically favorable patients treated with VC-SBRT only (group 1) and 80% for those who received also EBRT (group 2). No pelvic or vaginal recurrences were observed in either group. The 3-year actuarial DFS is 82.5% (90% and 67% for group 1 and 2, respectively), with disease progression exclusively occurring distantly, predominantly in the lung. Median survival has not yet been reached. SBRT was well tolerated and no acute/late urinary or gastrointestinal toxicities > grade 2 were observed.
Conclusion
Adjuvant vaginal SBRT CV appears to be a safe and effective alternative to BT for stage I-III endometrial cancer. Our preliminary analysis reveals excellent 3-year local control and tolerance. Larger prospective studies and longer follow-up are necessary to establish the role of vaginal SBRT as a new alternative standard of care.