Safety and feasibility of SBRT for elderly and frail patient with locally advanced bladder cancer
PO-1500
Abstract
Safety and feasibility of SBRT for elderly and frail patient with locally advanced bladder cancer
Authors: Giulia Marvaso1,2, Angelo Vitullo3,4, Riccardo Villa3,5, Giulia Corrao3, Maria Giulia Vincini3, Barbara Alicja Jereczek-Fossa3,5
1IEO, European Institute of Oncology IRCCS, Division of Radiation Oncology, Milan, Italy; 2University of Milan, Department of Oncology and Hemato-Oncology, Milan, Italy; 3IEO, European Institute of Oncology IRCCS, Division of Radiation Oncology , Milan, Italy; 4University of Milan, Department of Oncology and Hemato-Oncology, Milan, Italy; 5University of Milan, Department of Oncology and Hemato-Oncology , Milan, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
To report feasibility, oncological outcomes and toxicity rates of SBRT in a monocentric cohort of frail and elderly bladder cancer (BC) patients not eligible for curative treatments (cystectomy or trimodal therapy).
Material and Methods
Patients who underwent SBRT to the gross bladder tumor, or to the tumor bed after transurethral resection of bladder tumor (TURBT) at our Institution from 2017 to 2021 were retrospectively considered for study inclusion. IGRT SBRT with 6-MV photon X beams was delivered using three different linacs. Schedules of treatment were 30 and 25 Gy in 5 fractions (both every other day and consecutive days). Treatment response was assessed with radiological investigation and/or cystoscopy. Toxicity evaluation was carried out according to RTOG/EORTC v2.0 criteria.
Results
The study included 16 patients, 10 of them received SBRT on the gross bladder tumor (GT group) and 6 on the tumor bed after TURBT (TURBT group); altogether, 14 patients underwent previous local or systemic treatments. Baseline characteristics of the patients are shown in Table 1. Overall, low-grade (G) treatment toxicity was described, with one G1 and three G2 acute genitourinary toxicities reported and one G4 chronic toxicity in the GT group, that needed permanent vesical catheterization and then bilateral nephrostomy (that patient underwent two previous curative courses of RT to treat respectively prostate and rectal cancer); one G1 and one G2 acute gastrointestinal toxicities were reported. In the TURBT group no G>1 toxicity was reported. Patients’ response to treatment for patients with available follow-up (FU) was evaluated with cystoscopy in 6 patients and with a CT scan in 2 patients. In the GT group, at a median FU of 13.3 months (IQR 9.9-27.8), three complete responses to the treatment were reported; as of today, 50.0% of patients are alive; among them one patient had a complete response (CR), one patient had a local progression of the disease (PD) and another one experienced a systemic PD; 4 patients were lost at FU. For the TURBT group, at a median FU of 10.3 months (IQR 9.7-18.8) 4 out of 6 patients are alive with 3 CR, 1 stable disease, 1 local PD who needed 2 subsequent TURBT with concurrent intravesical BCG instillation, and 1 patient lost at FU; 1 patient was lost at FU.
Table 1: Summary of the patients’ characteristics
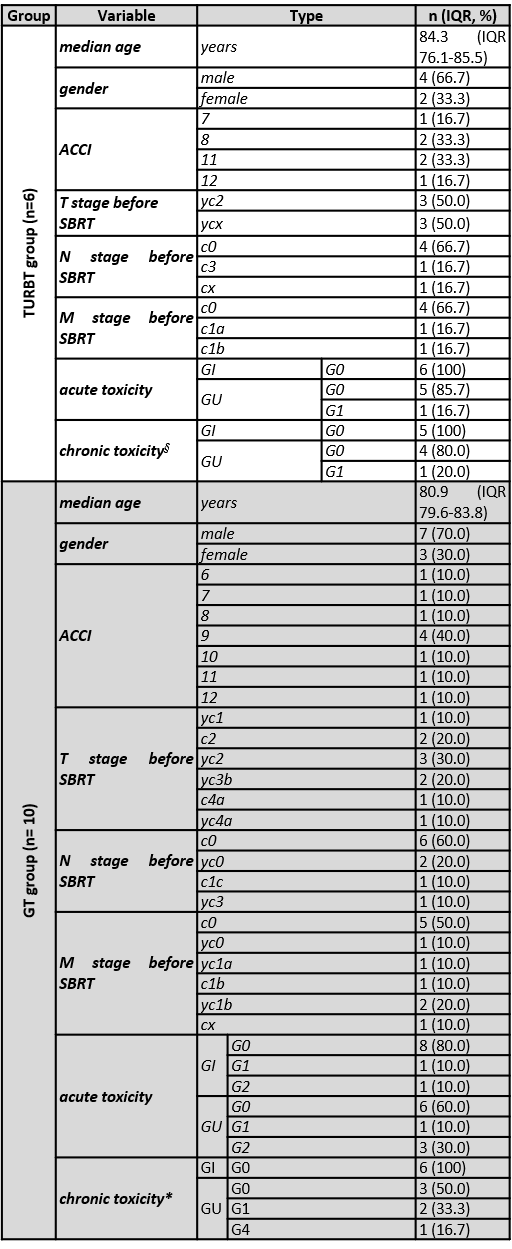
§ data available for five out of six patients; * data available for six out of ten patients.
Conclusion
To the best of our knowledge, we reported the first cohort of frail and elderly BC patients treated with SBRT to the GT and to the tumor bed after TURBT. Our preliminary data demonstrate that the treatment is technically feasible, with an acceptable toxicity profile. In this underrepresented setting of patients more solid indications are required based on further and updated FU data on toxicities and oncological outcomes.