The 20 eligible studies (RCT, 1: single-arm trial, 1; prospective cohort, 3; retrospective cohort, 15) from the Asia-Pacific (13), South America (3), North America (2), Europe (1), and Oceania (1), included cases treated from 1992 to 2016. Twelve were elderly cohorts, one, a renal failure cohort, and seven, with mixed contraindications (age, renal failure, poor PS, comorbidity, etc). Five interventions were represented: standard CRT (weekly cisplatin)(4); modified CRT (standard RT with ChT other than weekly cisplatin)(11); standard RT alone (11); modified RT (1); and standard ChT with modified RT (1). Nodal boost (NB) was used in 5 studies.
Pooled complete response (85%) and 5yOS (62%) rates are comparable to those published for LACC patients without contraindications. Subgroup analyses showed that 5yOS is better with ChT than none (73% vs 58%), and with NB than none (71% vs 56%), and lowest for carboplatin CRT (44%) despite better ChT compliance (86%). ChT compliance is better in renal failure than elderly cohorts (89% vs 67%). RT compliance is lower with ChT than none (90% vs 96%), and higher with NB than none (96% vs 93%). When ChT is given, NB is associated with lower RT compliance than no NB.
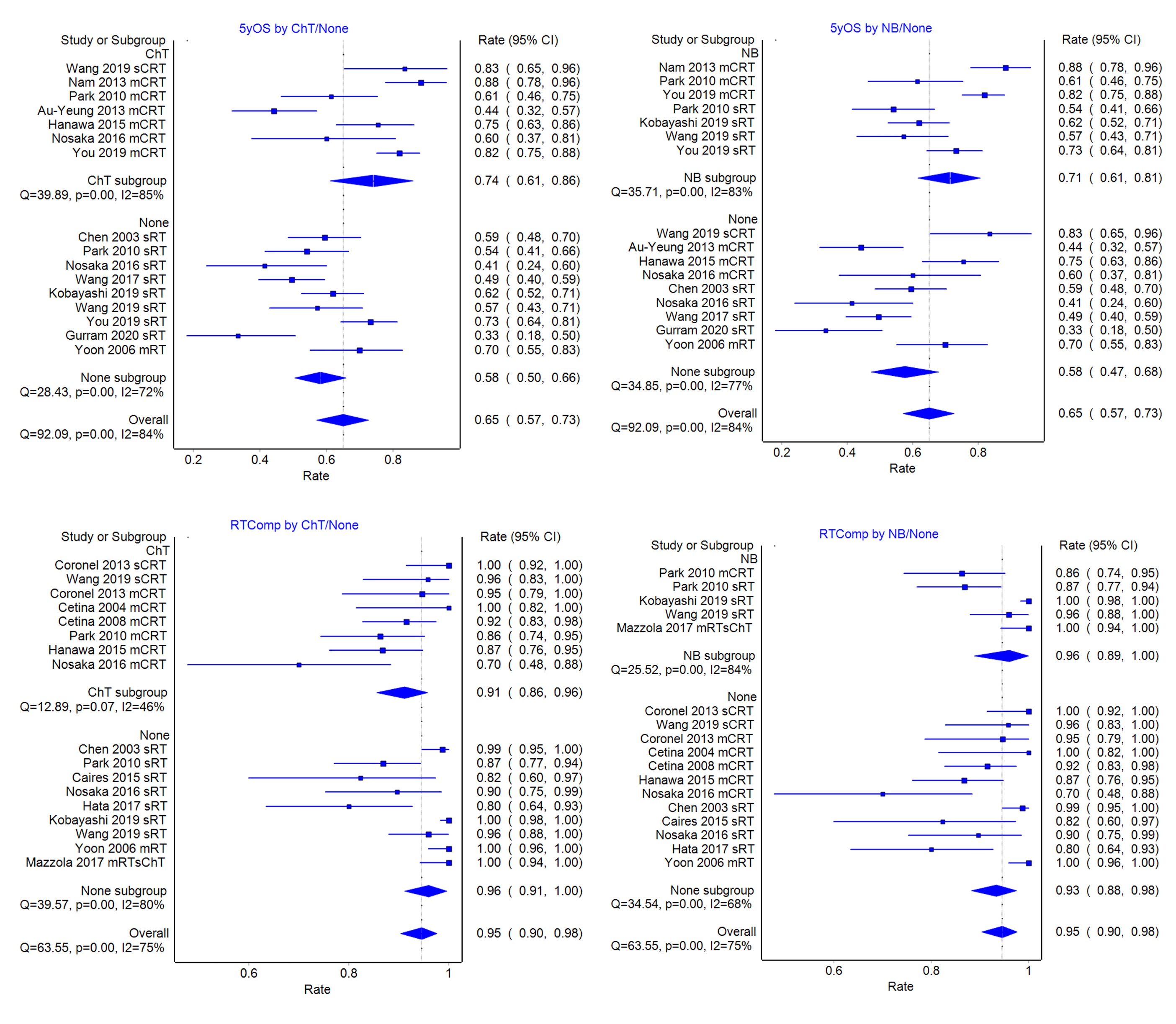
Meta-regression analyses confirm ChT and NB to be significant positive factors for survival, and NB, for RT compliance, partly due to its association with the use of advanced RT (3DCRT, IMRT) techniques.