Large primary tumours of oropharyngeal cancer may benefit from radiation dose escalation
PO-1240
Abstract
Large primary tumours of oropharyngeal cancer may benefit from radiation dose escalation
Authors: Anna Embring1, Eva Onjukka1, Claes Mercke1, Ingmar Lax1, Anders Berglund2, Signe Friesland1
1Karolinska Institutet, Department of Oncology-Pathology, Stockholm, Sweden; 2Epistat, Epistat, Uppsala, Sweden
Show Affiliations
Hide Affiliations
Purpose or Objective
Previous studies on radiation dose escalation have shown mixed results, and it is not established how to select the subgroup of head and neck cancer patients that would benefit from dose escalated radiotherapy. In this study we retrospectively analysed treatment outcome and side effects in patients with oropharyngeal cancer treated with dose escalated radiotherapy compared to a matched cohort treated with standard dose radiotherapy.
Material and Methods
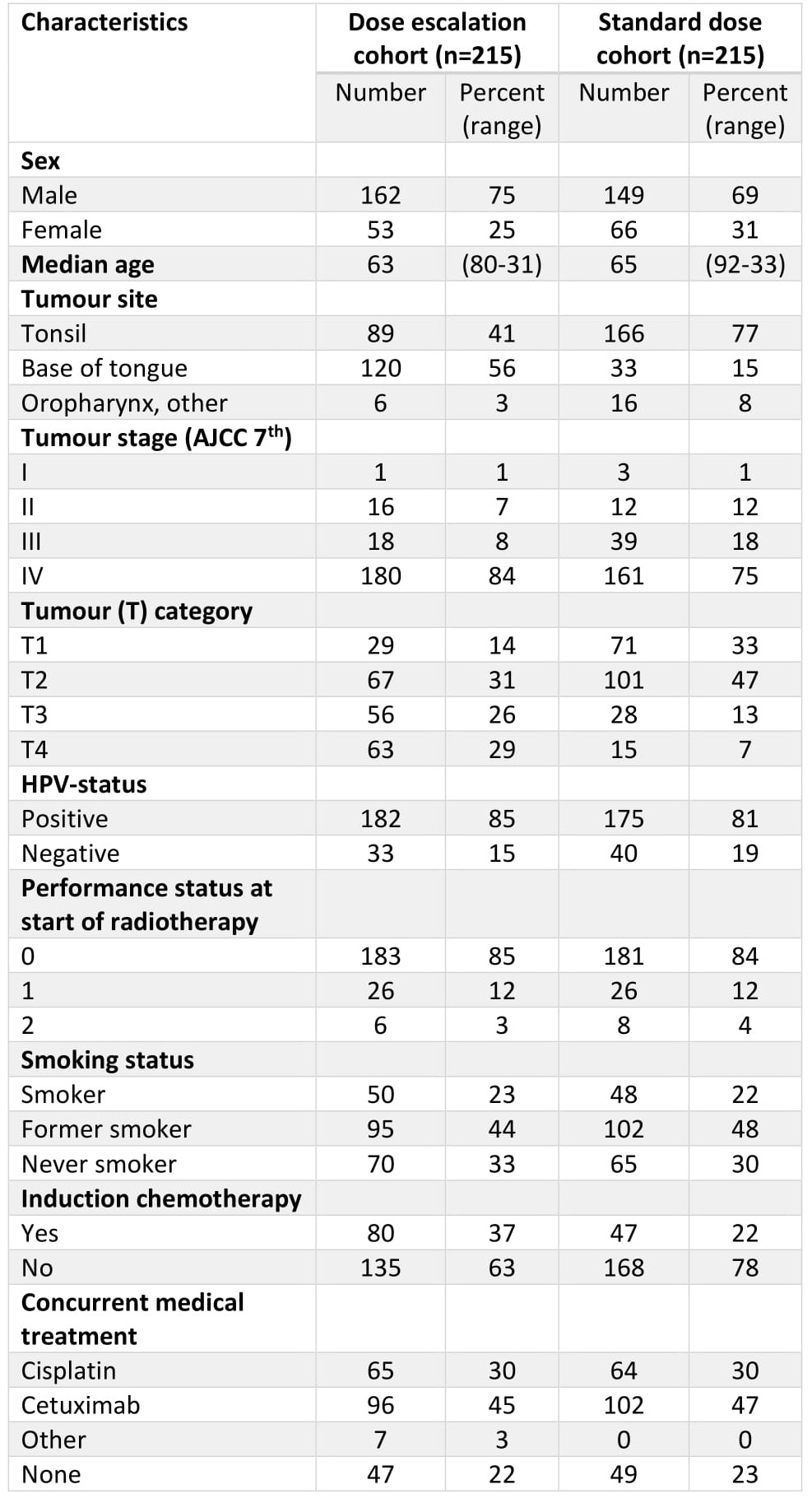
We identified 244 patients treated with >72 Gy (EQD2, α/β = 10 Gy) and 332 treated with 68 Gy for oropharyngeal squamous cell carcinoma between 2011 and 2018 at our institution. A matched cohort was selected for analysis, including 215 patients from each dose level. Data on treatment outcome and side effects (osteoradionecrosis (ORN), skin, mucosa, larynx, trismus, salivary glands and dysphagia) were collected from a local quality registry and supplemented with a review of medical records.
Results
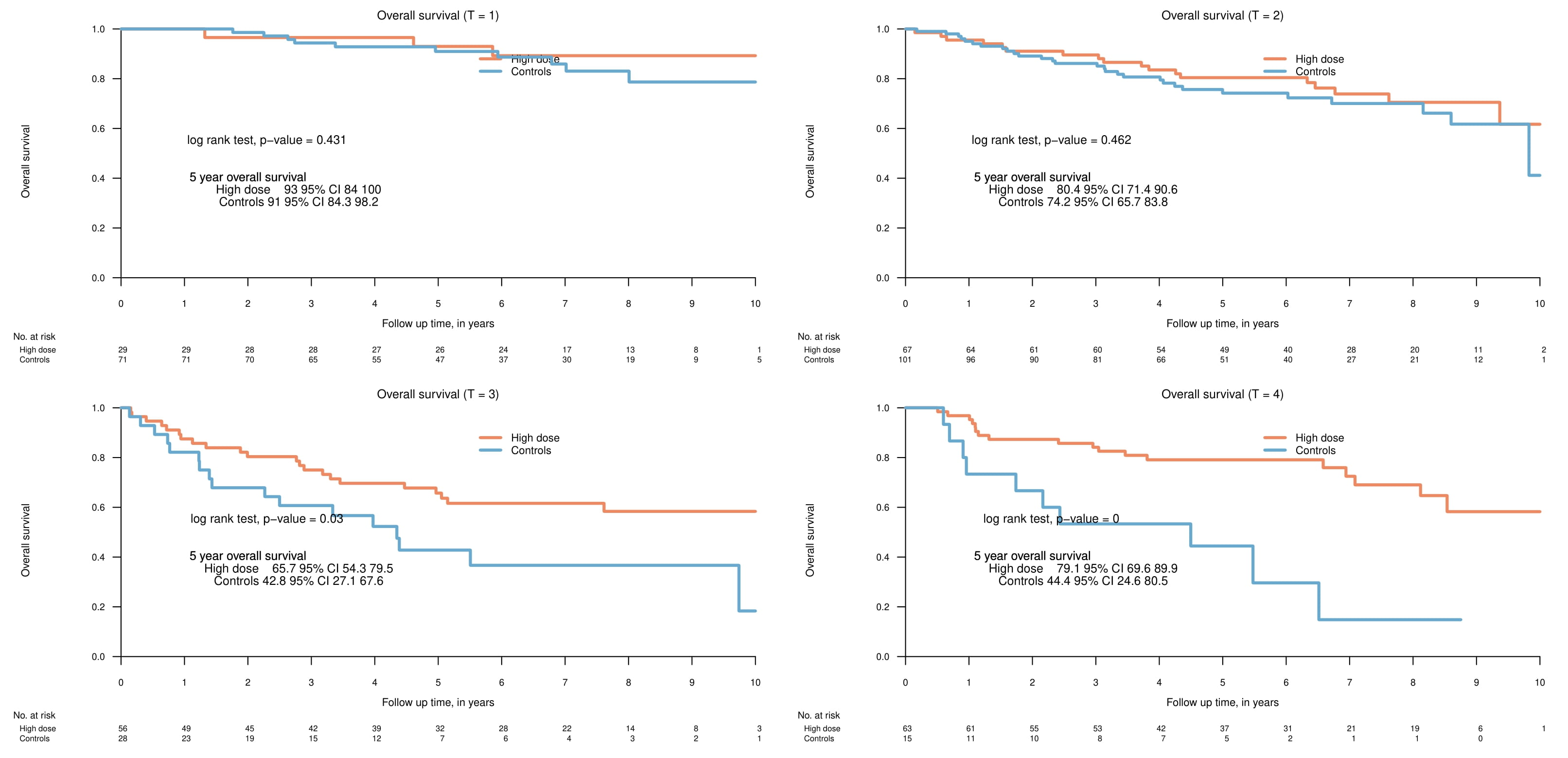
Clinical characteristics of the two subgroups are listed in Table 1. The 5-year overall survival (OS) was 77.8% (95% CI 72.4-91.6) and 73.7% (67.8-80.1) in the high dose and control group respectively (p=0.24). A subgroup analysis stratified by T-stage showed no significant differences in OS in patients with T1 or T2 tumours. By contrast, advanced primary tumours fared better in the high-dose subgroup with a 5-year OS in T3-tumours being 65.7% (54.3-79.5) and 42.8% (27.1-67.6) in the high-dose and control group respectively (p=0.03) and OS in T4-tumours being 79.1% (69.6-89.9) and 44.4% (24.6-80.5) in the high-dose and control group respectively (p<0.001). In accordance with this, no significant differences was seen in progression free survival (PFS) between the two groups in T1- and T2-tumours; while patients with advanced primary tumours in the high-dose group fared better with a 5-year PFS in T3-tumours of 66.6% (55.1-80.5), compared to 43.1% (27.4-67.8) in the control group (p=0.04), and similarly for T4-tumours with 71.4% (61.0-83.4) and 44.4% (24.6-80.5) in the high-dose and control groups, respectively (p=0.02). Grade ≥3 ORN and dysphagia were more common in the dose-escalated group compared to the control group, with 19 (8.8%) vs. 4 (1.9%) patients developing grade ≥3 ORN in the high-dose and control group, respectively (p=0.003), and 37 (17.2%) vs. 21 (9.8%) patients developing grade ≥3 dysphagia in the high-dose and control groups, respectively (p=0.04). There were no other significant differences in the investigated grade ≥3 side effects. Median follow up was 78.1 months in the dose-escalated cohort and 60.2 months in the control group.
Conclusion
Patients with small primary tumours do not benefit from radiation dose escalation, but patients with T3 and T4-tumours may benefit from dose escalated radiotherapy. Patients treated with dose-escalated radiotherapy have a higher incidence of certain grade ≥3 side effects compared to patients treated with standard-dose radiotherapy.