Metabolic reprogramming using OXPHOS inhibitors alleviates hypoxia in tumor spheroids
Anne Beerkens,
The Netherlands
PO-2212
Abstract
Metabolic reprogramming using OXPHOS inhibitors alleviates hypoxia in tumor spheroids
Authors: Anne Beerkens1,2, Daan Boreel1,2, Fleur Göllesch1, Pieter Roelofs1, Sandra Heskamp2, Gosse Adema1, Paul Span1, Johan Bussink1
1Radboud University Medical Center, Radiation Oncology, Nijmegen, The Netherlands; 2Radboud University Medical Center, Medical Imaging, Nijmegen, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Limited diffusion of oxygen into distant areas in combination with an increased oxygen consumption rate (OCR) leads to hypoxia in most solid tumors. Hypoxic areas in tumors are known to be radioresistant and can have a negative effect on the anti-tumor immune response. Recently, it became clear that tumor cells are also reliant on oxidative phosphorylation (OXPHOS) for their energy, opposing the longstanding notion that tumor cells primarily rely on glycolysis under aerobic conditions. Therefore, we aim to alleviate hypoxia through metabolic reprogramming by inhibition of the oxidative metabolism to sensitize tumor cells for radiotherapy, immunotherapy and its combination.
Material and Methods
Murine mouse colon MC38 cells containing a hypoxia response element upregulating eGFP under hypoxic conditions, were used to grow spheroids. 24h after spheroid formation, the spheroids were treated with different concentrations of the OXPHOS inhibitors IACS-010759, atovaquone and metformin. The eGFP signal for hypoxia was followed over time using the Incucyte Zoom Live Cell Imaging system. The hypoxic area and total spheroid are was determined and used to quantify the fraction of hypoxia.
Results
The data shows that hypoxic cores could be detected 24h after spheroid formation. Inhibiting OXPHOS with 1 µM IACS-010759 alleviates hypoxia in spheroids, compared to the control spheroids (0 µM IACS-010759), shown by a reduction in the green signal within 30 min after start of treatment, which persisted for several hours (Fig. 1A). Furthermore, the fraction hypoxia revealed that 1 µM IACS-010759, as well as 0.33 µM IACS-010759 were able to alleviate hypoxia, while 0.11 or 0.037 µM IACS-010759 were unable to alleviate hypoxia in the center of the tumor spheroids. Spheroids treated with atovaquone (30 µM) and metformin (3 mM or 9 mM) showed similar results. In addition, a dose-dependent response was observed after treatment with IACS-010759, atovaquone and metformin (Fig. 1B).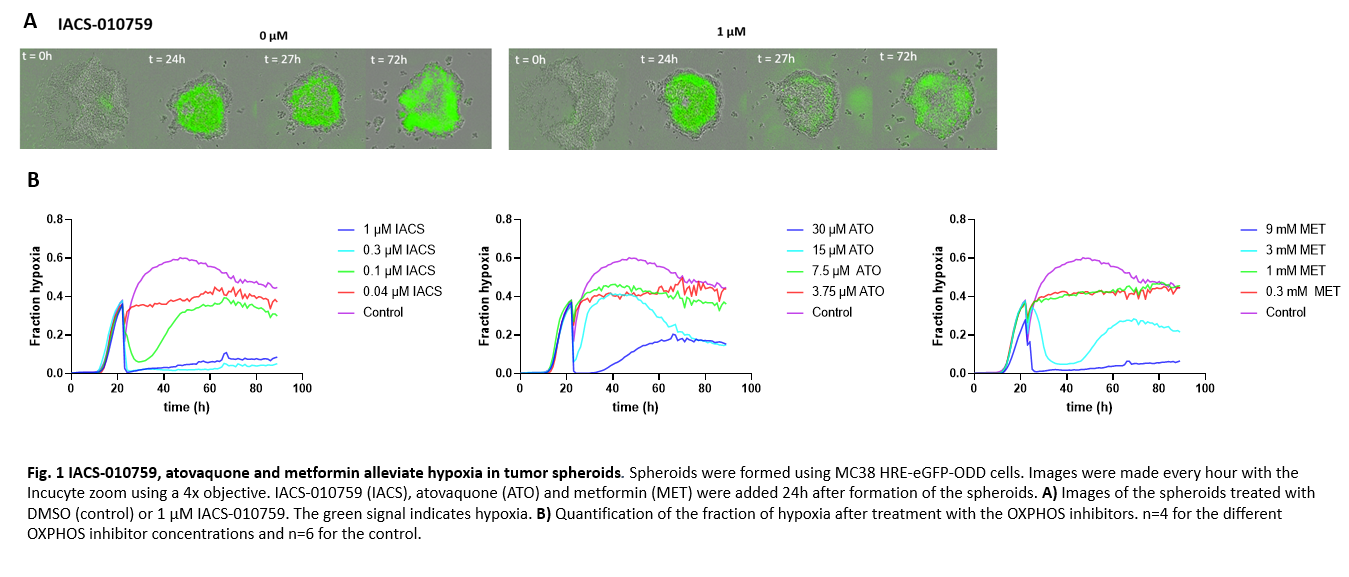
Conclusion
We developed an in vitro model in which chronic hypoxia can be quantified over time using a Live Cell Imaging system. Using this system, we can determine the effect of several OXPHOS inhibitors on hypoxia alleviation. We showed that the OXPHOS inhibitors IACS-010759, atovaquone and metformin could alleviate hypoxia within 30 minutes after start of treatment and this sustained for several days in a dose-dependent manner. The observed reduction in hypoxia might sensitize hypoxic tumor cells for radiotherapy, immune checkpoint blockade and their combination.