One-Week External Beam Partial Breast Irradiation: Survival and Toxicity Outcomes
Riccardo Ray Colciago,
Italy
PD-0233
Abstract
One-Week External Beam Partial Breast Irradiation: Survival and Toxicity Outcomes
Authors: Riccardo Ray Colciago1, Eliana La Rocca1, Carlotta Giandini1, Alicia Rejas Mateo1, Giuseppe Capri2, Secondo Folli3, Nice Bedini1, Laura Lozza1, Silvia Meroni4, Emanuele Pignoli4, Tiziana Rancati5, Stefano Arcangeli6, Maria Carmen De Santis7
1Fondazione IRCCS Istituto Nazionale dei Tumori, Radiotherapy, Milan, Italy; 2Fondazione IRCCS Istituto Nazionale dei Tumori, Medical Oncology, Milan, Italy; 3Fondazione IRCCS Istituto Nazionale dei Tumori, Breast Unit, Milan, Italy; 4Fondazione IRCCS Istituto Nazionale dei Tumori, Medical Physics Unit, Milan, Italy; 5Fondazione IRCCS Istituto Nazionale dei Tumori, Prostate Cancer Program, Milan, Italy; 6Ospedale San Gerardo, Radiation Oncology, Monza, Italy; 7Fondazione IRCCS Istituto Nazionale dei Tumori , Radiotherapy, Milan, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
According to ASTRO and ESTRO guidelines, external beam Partial Breast Irradiation (PBI) is a valid option for early-stage Breast Cancer patients. Nevertheless, there is still a lack of consensus about the best treatment schedule. In this study, we aimed to collect data from patients who underwent PBI in one week.
Material and Methods
We retrospectively analyzed survival and toxicity data of female patients treated in our institution from 2013 to 2022. Inclusion criteria were age ≥ 50 years, conservative breast surgery for non-lobular, G < 3, and luminal-like breast cancer with a maximum diameter of 20 mm without lymph nodal invasion. Gross Tumor Volume (GTV) was defined as the breast tissue between surgical clips; Clinical Target Volume (CTV) was an isometric expansion of 15 mm from the GTV, cropped into the homolateral breast; Planned Target Volume (PTV) was CTV + 5 mm of isometric expansion. The treatment schedule was 30 Gy delivered with VMAT in 5 daily fractions, prescribed at 95% of the dose to the PTV. ConeBeam Computed Tomography was used for IGRT.
The primary endpoint was Local Control (LC). Disease Free Survival (DFS), Overall Survival (OS) and safety were secondary outcomes. Toxicities and cosmesis were analyzed sec. RTOG and Harvard scales, respectively.
Results
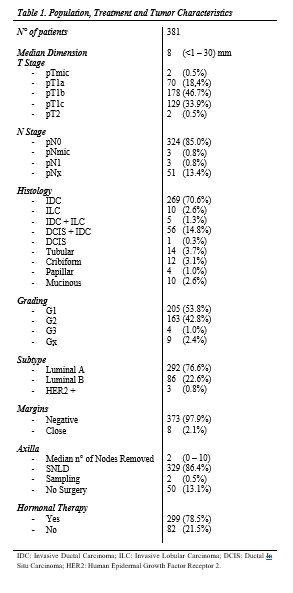
Three hundred eighty-one patients with a median age of 69 (33 – 91) years were included in the study. Characteristics are summarized in Table1. One hundred ninety-two (50.4%) of the cases were left breast; 360 (94.5%) were invasive ductal carcinomas, 10 (2.6%) were lobular invasive carcinomas, 10 (2.6%) were other histologies, and 1 (0.3%) was ductal in situ carcinoma; 368 (96.6%) were G1 or 2, and 4 (1.0%) were G3. Median dimension of the lesions was 8 mm (<1 – 30); 292 (76.6%), 86 (22.6%) and 3 (0.8%) were Luminal A, B and HER2-positive, respectively.
After a median follow-up of 28 (6 – 104) months, 7 (2.0%) experienced local recurrence, of which two were “In field”. Among local relapses, 6 (85.7%) were not prescribed hormonal therapy, 1 (14.3%) did not complete radiation therapy, and 1 (14.3%) had an underpowered covering of the PTV (= 94.3%). Three-year LC, DFS and OS were 97.5% (95% C.I. 96.2% – 98.8%), and 96.9% (95% C.I. 95.7% – 98.1%) respectively.
No ≥ G3 acute nor late toxicities were reported. There were 8 (2.1%) cases of G2 acute fibrosis, and 10 (2.9%) patients experienced G2 late toxicities. Five (1.5%) patients reported late cardiac major events, but 4 (80%) of them were treated on the right breast. Three (0.9%) late pulmonary toxicities were detected. Good or Excellent cosmetic evaluation sec. Harvard Scale was assessed in 252 (96.9%) cases by the physician VS 241 (89.2%) cases by the patients.
Conclusion
Our dose prescription is the most sustainable choice for patients and hospitals. This study demonstrated that “One-week” PBI is also effective and safe.