Skin Dose Predictors of Early-Late Reactions after Whole Breast RT in a Single-Center Large-Cohort
Alessandro Cicchetti,
Italy
PD-0232
Abstract
Skin Dose Predictors of Early-Late Reactions after Whole Breast RT in a Single-Center Large-Cohort
Authors: Alessandro Cicchetti1,2, Andrei Fodor3, Paola Mangili2, Martina Mori2, Anna Chiara4, Chiara Deantoni3, Marcella Pasetti3, Gabriele Palazzo2, Maria Giulia Ubeira-Gabellin5, Tiziana Rancati6, Antonella Del Vecchio2, Nadia Gisella Di Muzio3,3, Claudio Fiorino2
1Fondazione IRCCS Istituto Nazionale dei Tumori di Milano, Prostate Cancer Program, Milan, Italy; 2San Raffaele Scientific Institute, Medical Physics, Milan, Italy; 3San Raffaele Scientific Institute, Radiotherapy, Milan, Italy; 4San Raffaele Scientific Institute, Radiotherapy, milan, Italy; 5San Raffaele Scientific Institute, Medical, Physics, Italy; 6Fondazione IRCCS Istituto Nazionale dei Tumori di Miano, Prostate Cancer Program, Milan, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
To assess skin dosimetry predictors of local oedema and hyperpigmentation (EH) in breast cancer patients treated with whole breast moderately hypo-fractionated radiotherapy.
Material and Methods
We explored for the first time the possible causality between the damage to the cutaneous tissue and the EH endpoints through dosimetric descriptors of the skin. The skin structure was defined as the first 5 mm body layers and was retrospectively segmented on a large cohort of early-stage breast cancer patients treated between 2009 and 2017. Automatic delineation, followed by the extraction of skin DVH, was accomplished by scripting using the MIM_assistant software. Patients were treated with tangential field RT, delivering 40 Gy in 15 fractions to the whole breast and without any dose boost. Radiation oncologists reported toxicity scores during FU according to the SOMA-LENT scale . The study endpoint was moderate-severe Oedema-Hyperpigmentation (EH G2+). NTCP model using logistic regression was developed by combining dosimetric and clinical factors.
Results
EH was assessed considering previous findings showing an incidence plateau for EH after six months. A total of 52 (4.7%) over 1118 patients (with complete dosimetry and clinical information available and at least six months FU) experienced EH G2+, i.e., marked or generalized hyperpigmentation or moderate localized oedema, and intervention indicated or limited instrumental activity of daily living.
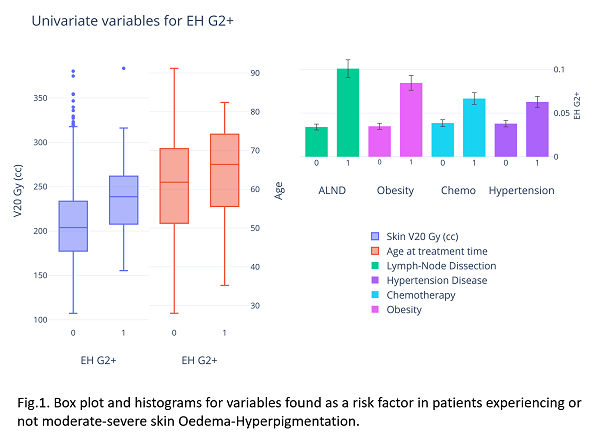
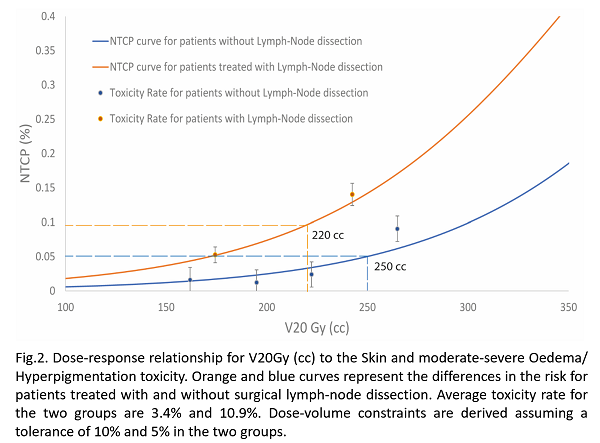
Box plots and histograms are depicted for the most significant factors at univariate analysis (See Fig.1.). A two-variable model was identified, including absolute V20Gy to the skin (50% of the prescribed dose) and axillary lymph node dissection (ALND), with a moderate AUC (0.74 CI:0.02, p<0.0001). For this risk factor, the rate of EH G2+ was 3.4% vs 10.1% (OR = 3.1 and p<0.001), while the median V20 Gy value for EH G0-1 vs G2+ patients was 204 cc vs 239 cc. The dose-response curves for patients with and without ALND are shown in Fig.2. From the NTCP model, we could identify V20Gy<250cc for an EH risk below 5% for patients without ALND. In the ALND group, we could apply a constraint of 220 cc for the risk of EH below 10%. Those conditions were currently satisfied in 86% and 31% of patients, confirming the strong impact of ALND.
Conclusion
The association between moderate-severe Oedema-Hyperpigmentation and skin DVH was quantified. For the first time to our knowledge, the current study identified dose-volume warnings for a structure usually not considered during plan optimization. Our findings should help planners to obtain more conformal dose distributions, when feasible, in high-risk patients with large breast. Indeed, the dose-volume effect was confirmed in patients previously submitted to ALND, although these show about a three-fold risk compared to patients without ALND.
The study was funded by AIRC IG 25951 – 23150 and Fondazione Regionale per la Ricerca Biomedica project nr. 110-JTC PerPlanRT ERA PerMed, GA 779282.