Ex vivo response markers for radiotherapy treatment personalization of oropharyngeal cancer patients
Iris Lauwers,
The Netherlands
PO-2231
Abstract
Ex vivo response markers for radiotherapy treatment personalization of oropharyngeal cancer patients
Authors: Iris Lauwers1, Katrin Pachler2, Gerda Verduijn1, Aniel Sewnaik3, Jose Hardillo3, Dominiek Monserez3, Stijn Keereweer4, Dik van Gent2, Mischa Hoogeman1,5, Marta Capala1, Steven Petit1
1Erasmus MC Cancer Institute, University Medical Center Rotterdam, Department of Radiotherapy, Rotterdam, The Netherlands; 2University Medical Center Rotterdam, Department of Molecular Genetics, Rotterdam, The Netherlands; 3University Medical Center Rotterdam, Department of Otorhinolaryngology, Rotterdam, The Netherlands; 4University Medical Center Rotterdam, Deparment of Otorhinolaryngology, Rotterdam, The Netherlands; 5HollandPTC, Department of Medical Physics and Informatics, Delft, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Locoregional failure rate remains high in oropharyngeal squamous cell carcinoma (OPSCC) patients, while long-term toxicity is substantial. Therefore, OPSCC patients would benefit from personalized treatments. The first step towards personalization is patient stratification in responders and non-responders, before or early during treatment. We hypothesize that ex vivo tumor irradiation response could be predictive for clinical radiotherapy response. However, it is not clear which ex vivo parameter best reflects clinical response. A logical candidate is apoptosis induction measured 5 days after radiation.[1] However, for personalized treatment selection a faster read out would be desirable. Therefore, the aim of this study was to investigate earlier ex vivo irradiation response markers, using apoptosis induction at 5 days post-irradiation as benchmark.
Material and Methods
OPSCC patients of the BIO-ROC study (NL8450) treated between 14/3/2022 and 18/8/2022 were included. Fresh OPSCC biopsy samples derived at the outpatient clinic were sliced, cultured, irradiated (5Gy), and fixed after 24h or 5d post-irradiation. Apoptosis (TUNEL) was imaged using fluorescence microscopy and quantified as the percentage of the total nuclear area (DAPI). DNA repair kinetics were analyzed by residual 53BP1 foci quantification in tumor nuclei (immune-stained with p63) using fluorescence confocal microscopy. The foci count per nuclear tumor volume was automatically determined using a deep learning model.[2] Automatic images analysis enabled analysis of multiple field of views and over 100 nuclei per condition. Apoptosis and DNA repair kinetics at 24h were considered as potential fast readout response markers and apoptosis at 5d was used as benchmark. To determine the effect of radiation, the outcome of the treated condition relative to control condition was calculated. Since two year follow up was not achieved yet for all patients, as surrogate for treatment response, the benchmark apoptosis at 5d was compared between HPV positive and negative patients, of which the latter are known to have unfavorable clinical outcomes.[3]
Results
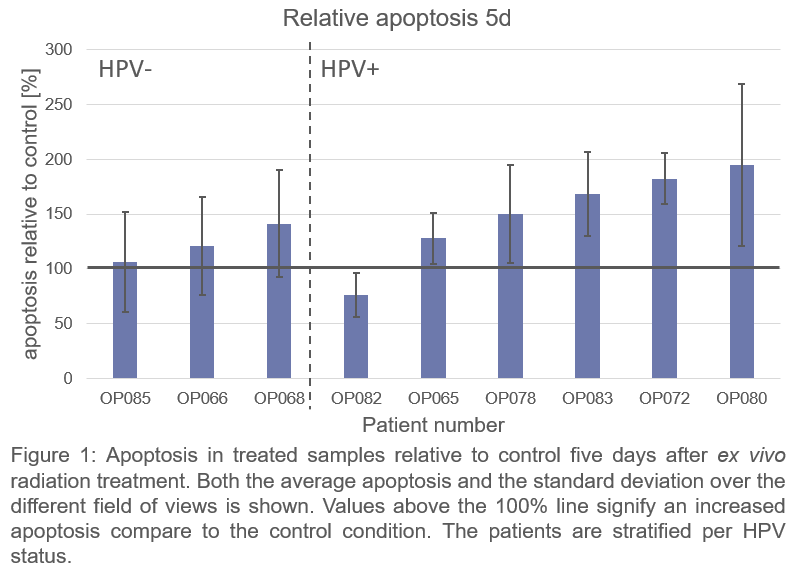
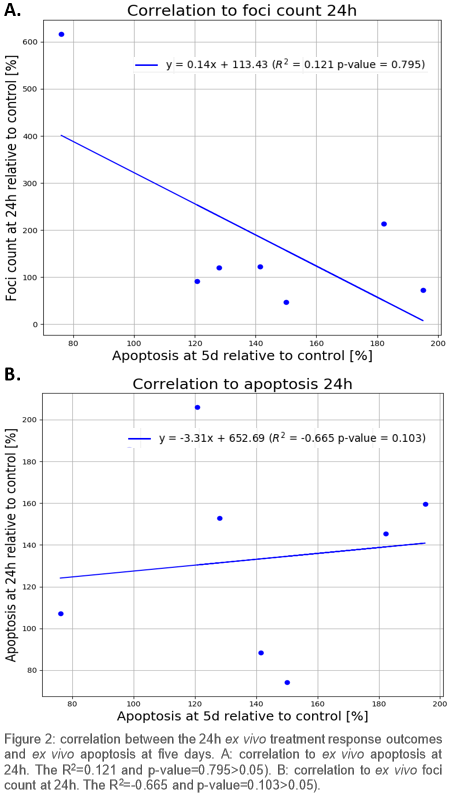
Nine patients were included. On average more radiation-induced apoptosis was found in HPV-positive compared to HPV-negative OPSCC patients at 5d (Fig 1), which indicates that apoptosis at 5d is related to expected radiosensitivity differences. Neither apoptosis nor DNA repair kinetics at 24h correlated strongly with apoptosis at 5d (p>0.05) (Fig 2).
Conclusion
Residual DNA damage did not correlate to more apoptosis; therefore, residual DNA damage might not be suitable to predict radiosensitivity. Moreover 24h after treatment appears to be too early to measure radiation-induced apoptosis, as absolute apoptosis at 24 hours was low and did not correlate to apoptosis at 5d. Apoptosis at 5d, however, did reflect the radiosensitivity differences that were expected based on HPV status.
1 ME Capala (2021) Oral Oncology
2 I Lauwers (2022) ESTRO
3 KK Ang (2010) New England Journal of Medicine