Poly-ether-ether-ketone: An improved bone replacement material compliant with proton radiotherapy
PO-1979
Abstract
Poly-ether-ether-ketone: An improved bone replacement material compliant with proton radiotherapy
Authors: Georgio Katsifis1, Matthew Jennings2, David McKenzie1, Michael Jackson3, Natalka Suchowerska1
1The University of Sydney, VectorLAB, School of Physics, Sydney, Australia; 2Townsville Hospital and Health Service, Department of Medical Physics, Townsville, Australia; 3Prince of Wales Hospital, Department of Radiation Oncology, Sydney, Australia
Show Affiliations
Hide Affiliations
Purpose or Objective
Patients presenting for radiation therapy with metallic implants require specific attention in treatment planning. Although implant avoidance and correction algorithms have offered workable, but not always accurate solutions for photon beams [1, 2], in proton beams, the higher density and atomic number implant materials can cause a significant shift in the Bragg Peak and consequently the dose distribution [3]. This perturbation results in overdosing healthy tissue and underdosing the target (~ +/- 25%) [3]. An innovative alternate approach is to change the implant material to Poly-ether-ether-ketone (PEEK): a thermoplastic polymer with a density and stopping power close to water, which we have shown to have material properties viable for use as bone replacement [4]. In this work we demonstrate the effect of a PEEK implant on the dose distribution from multiple proton beams using both Monte Carlo methods and radiation treatment planning systems.
Material and Methods
Monte Carlo simulations using GATE/Geant4 [5] were used to determine the effect of an implant, designated to water, PEEK or titanium, on the dose distribution when exposed to a proton beam of energies (E_0=80,130,200), incident on a 30 cm water phantom with a 5 mm PEEK/titanium implant placed at a depth of 50 mm (Figure 1).
A parallel study performed using the beam data of a proton treatment system calculates the dose distribution on a patient with an implant, which is designated to be either bone, titanium or PEEK. In each case the implant is either in the beam, partially in the beam or next to the beam, and repeated with positional displacements.
Results
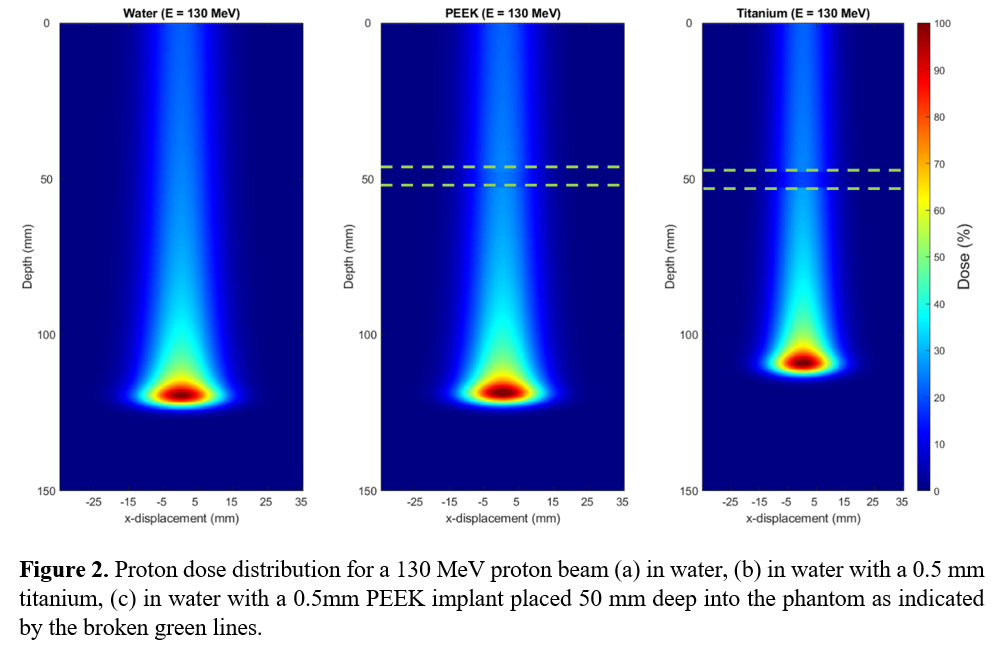
Figure 1 shows the depth dose curves for a proton beam incident on the 30 cm cube phantom. When the implant is titanium, the Bragg Peak shifts towards the entrance surface but is influenced by the location of the implant relative to the position of its Bragg Peak. The peak dose for titanium shifted slightly for proton energies of 130 and 200 MeV with a more significant shift at 80 MeV, where the Bragg Peak occurred within the titanium implant. Figure 2 depicts the 2D dose profile for a 130 MeV proton beam for PEEK/titanium implants, indicating a reduction in range from the titanium implant.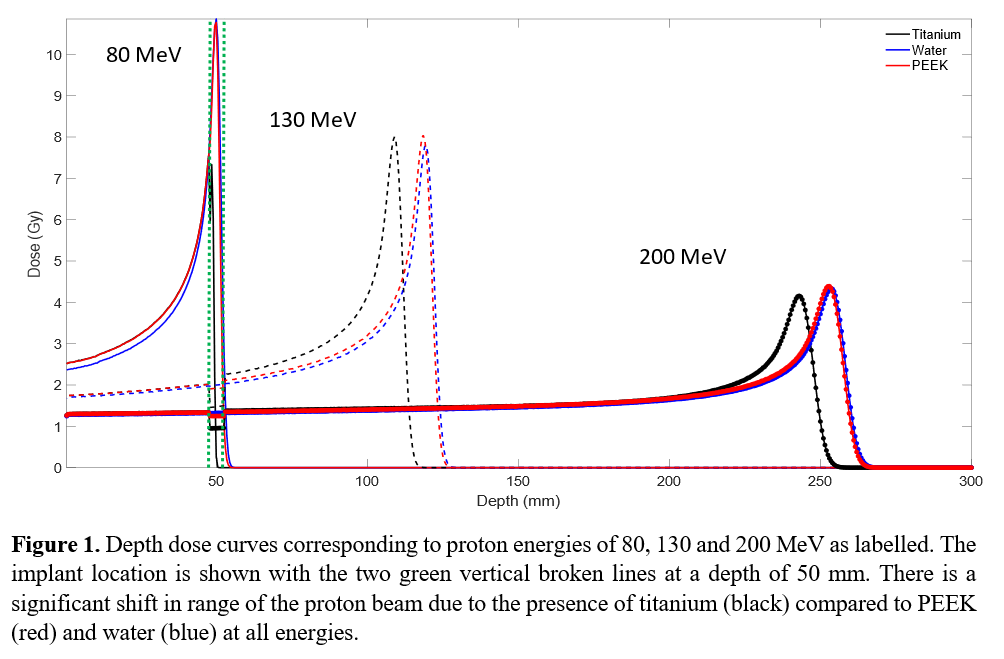
Conclusion
Titanium implants perturb the proton dose distribution, significantly reducing the robustness of the treatment plan. PEEK was found to shift the range of the Bragg Peak and perturb the dose, relative to water, by less than 1.6 % and 2 % respectively. PEEK is demonstrated to be a favourable implant material for proton therapy.
The use of PEEK as an alternative to titanium for cancer patients directly addresses the core of the problem in imaging and dosimetry introduced by using high density material in radiation beams. PEEK implants preclude the need for additional image processing, implant avoidance and dose correction processes. PEEK implants might enable patients to undergo proton treatment that would have otherwise been impossible due to the presence of metallic implants.