Intra-tumoral necrosis in Head and Neck proton and carbon ion therapy: the role of Dose, RBE and LET
PO-1977
Abstract
Intra-tumoral necrosis in Head and Neck proton and carbon ion therapy: the role of Dose, RBE and LET
Authors: Silvia Molinelli1, Maria Bonora2, Alessandro Vai1, Sara Ronchi2, Rossana Ingargiola2, Anna Maria Camarda2, Nadia Facchinetti2, Mario Ciocca1, Barbara Vischioni2, Ester Orlandi2
1Fondazione CNAO, Medical Physics, Pavia, Italy; 2Fondazione CNAO, Clinical department, Pavia, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
To understand the potential role of RBE-weighted dose (DRBE), RBE modelling and dose-averaged LET (LETd) in the development of intra-tumoral necrosis after proton (PT) and carbon-ion (CIRT) treatment of head and neck (HN) tumours.
Material and Methods
For this analysis we considered all HN cases, definitively treated with PT or CIRT between 2013 and January 2022, free of local recurrence during follow-up, which experienced soft tissue necrosis starting at the treated tumor site (STN).
The STN volume was contoured on the planning CT, rigidly registered with MRI study showing STN at the first occurrence. PT plans were recalculated with RBE=1.1 and the McNamara model with variable a/b ratio (2 and 10 Gy); CIRT plans with the local effect (LEM) and microdosimetric kinetic model (MKM). DRBE and LETd distributions in the STN were analysed in comparison with GTV values, looking at percentage deviation in near-to-maximum (D1%) and median DRBE (D50%), for each RBE model, and LETd (L1%, L50%) values. We finally analysed the incidence of STN, per particle type, in relation to fractionation schedule variations during the study period.
Results
Five PT and 9 CIRT cases developed SNT at a median follow up of 6 and 16 months, respectively. All patients, except one CIRT case, had adenoid cystic carcinomas (ACC). In total, 152 ACC patients received definitive CIRT; while the PT protocol was activated in 2017, with 54 patients treated to date. In the STN group, the median prescription dose was 69.96 Gy(RBE) (range 66-70) in 33 fractions for PT and 68.8 Gy(RBE) (65.6; 70.4) in 16 fractions for CIRT.
DRBE was not significantly different in the STN with respect to the GTV, (median D1%= -0.1% [-0.2%; 0.1%] for PT; 0.6% [-0.8%;0.7%] for CIRT), with no influence of RBE modelling. LETd was lower (median L1%= 10.6% [-14.1%;-6.1%] for PT; -13.0% [-17.4%;-11.4%] for CIRT), being the necrotic area mainly located in the target centre (Figure 1).
In consideration of the annual incidence of SNT after CIRT in 2015 (6%), the prescription dose was reduced to 65.6 Gy(RBE). The initial PT scheme (1,8-2 Gy(RBE) per fraction) was implemented in 2017 for ACC invading large part of the oropharyngeal mucosa infiltration. The subsequent introduction of a moderate hypofractionated schedule (2.12 Gy(RBE)) could have been one of the factors contributing to the increase of the PT toxicity rate (7%) for this critical group. Assuming a higher sensitivity of the involved tissue, the McNamara (a/b 2 Gy)-based calculation indicated a 9.5% increase in the median target DRBE, which would correspond to 2.32 Gy(RBE) per fraction.
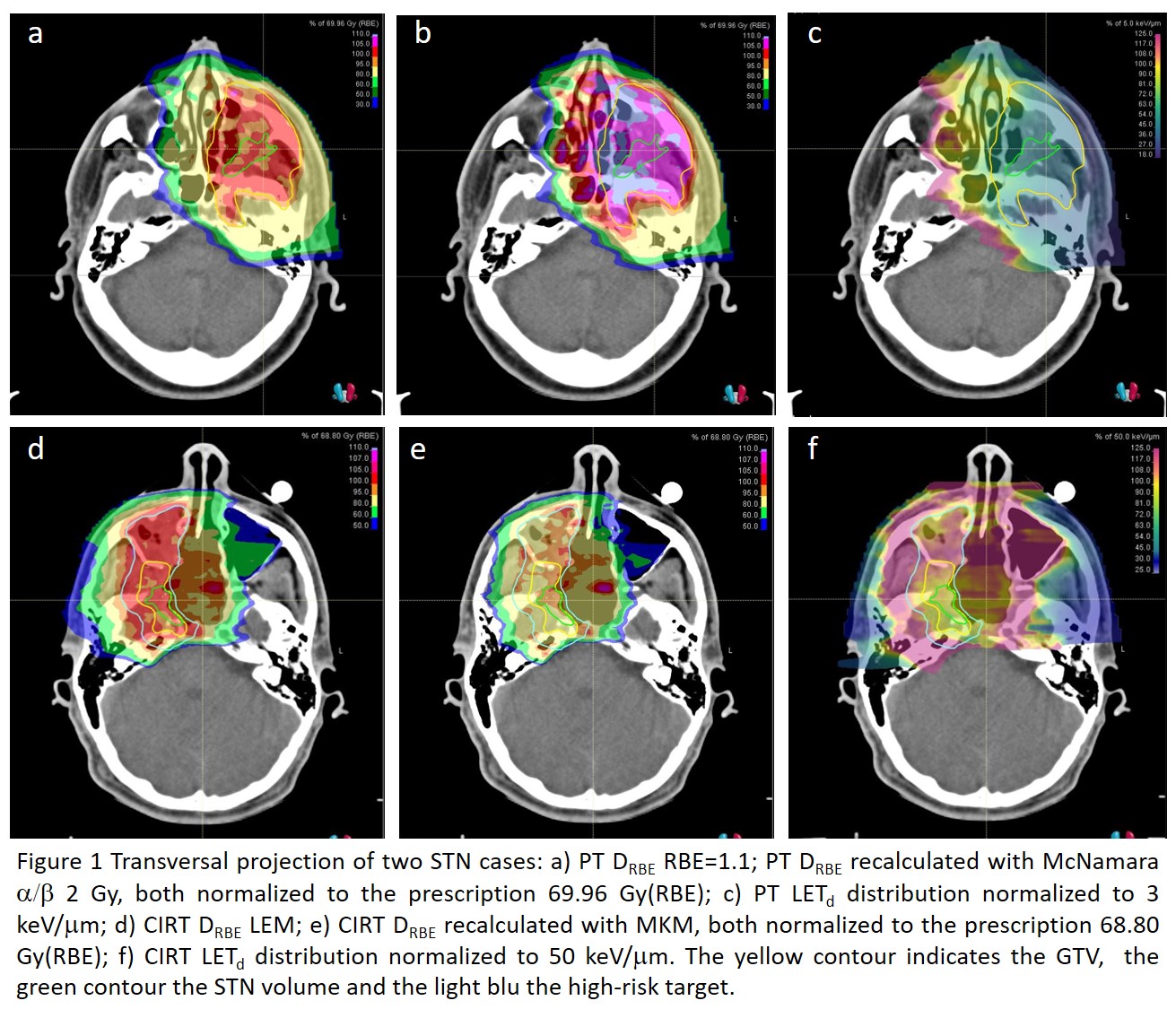
Conclusion
Our preliminary results seem to find a relationship between STN and tumor histology and the prescribed dose and fractionation schedule, for both PT and CIRT. Further analyses are mandatory to confirm our findings, including evaluation of potential clinical dose-modifying factors, histological subtype and individual patient radiosensitivity profiles, possibly in comparison with a control patient pool.