Microdosimetric approach for treatment planning in proton therapy
Pietro Pisciotta,
The Netherlands
PO-1969
Abstract
Microdosimetric approach for treatment planning in proton therapy
Authors: Pietro Pisciotta1, Jacopo Magini2, Erik Traneus3, Mohammad Hussein4, Stefan Both1, Giuseppe Schettino2, Francesco Romano5
1University Medical Center Groningen, Department of Radiotherapy, Groningen, The Netherlands; 2University of Surrey, Department of Physics, Guildford, United Kingdom; 3RaySearch Laboratories, -, Stockholm, Sweden; 4National Physical Laboratory, -, Teddington, United Kingdom; 5Istituto Nazionale di Fisica Nucleare, Catania Division, Catania, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
The use of stochastic measurable microdosimetric quantities, like the energy deposition at a micrometric scale, can lead to a better radiobiological prediction for densely ionizing radiation such as protons. Accurate microdosimetric calculation can currently only be achieved with general purpose Monte Carlo simulation which is extremely slow, limiting clinical adoption. To improve the calculation accuracy within a clinical TPS, workaround need to be elaborated. This work attempts to implement microdosimetric quantities for proton treatment planning via look-up tables(LUTs) to be linked with a TPS. This is of fundamental importance in the view of achieving biological optimization through the microdosimetric kinetic model (MKM) or similar based on microdosimetric means, in a TPS.
Material and Methods
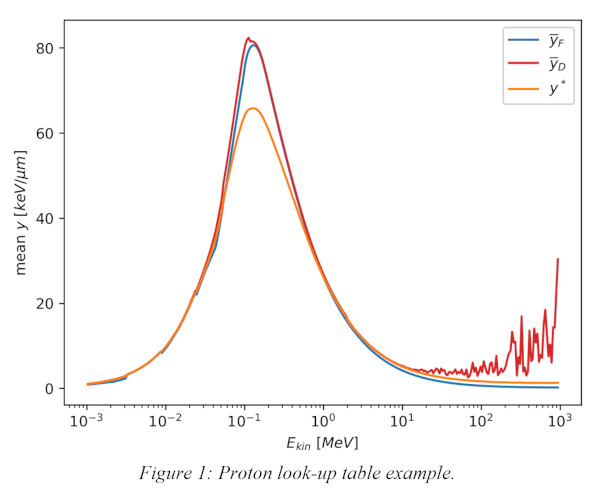
The LUTs include the microdosimetric mean quantities(ȳD, ȳF and y*) of monoenergetic proton beams as a function of their kinetic energy. These tables were obtained from a monoenergetic beam impinging perpendicularly on a 1µm thick sensitive volume using Geant4. Kinetic energy values were sampled in a logarithmic scale and associated with ȳD, ȳF and y*.
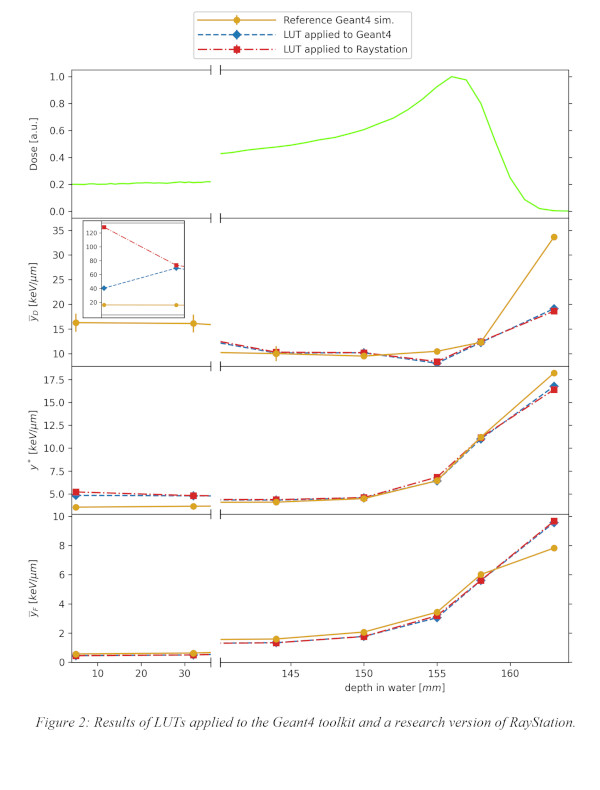
As a first step, the consistency of the proposed approach was validated by determining ȳD, ȳF and y* at different depths in water, using the previously calculated LUTs. The obtained quantities were compared with those retrieved at the same depths in water using a “full” Geant4 simulation, considered as a reference. To achieve this, a 150MeV monoenergetic proton beam was simulated on a water phantom and the kinetic energy distribution per voxel was scored, tracking only protons and heavier hadrons and excluding secondary electrons. LUTs were applied to each distribution, giving the overall ȳD, ȳF and y* for that voxel. As a second step, the same approach was repeated in a research version of the RayStation TPS(v11B IonPG), the LUTs were applied and, again, the obtained results were compared with the reference Geant4 simulation to validate the procedure in a TPS.
Results
A set of LUTs has been built for protons(Fig.1). Their validity has been tested using Geant4 and the results are in good agreement(Fig.2). The agreement between ȳD, ȳF and y* obtained by applying the LUTs to the Geant4-obtained kinetic energy distribution (blue) and those calculated directly from the microdosimetric spectrum using the reference Geant4 simulation(orange) is within 10% throughout the whole Bragg curve. The only exception are voxels in the entrance channel, whose much higher discrepancies are being investigated. Moreover, the LUTs method applied using RayStation(red) with a setup configuration similar to that implemented in Geant4 shows good agreement(Fig.2).
Conclusion
This study has demonstrated the feasibility of using LUTs to calculate microdosimetric quantities in a TPS that are comparable with Geant4. This is the first step in the implementation of biological models based on microdosimetry, such as MKM, within a TPS using microdosimetric quantities.