Robust LET optimization of proton arcs can substantially reduce high LET in critical structures
PO-1948
Abstract
Robust LET optimization of proton arcs can substantially reduce high LET in critical structures
Authors: Lars Glimelius1, Otte Marthin1, Viktor Wase2, Johan Sundström2, Erik Engwall1, Albin Fredriksson3, Henrik Melbéus1, Fredrik Tamm1, Björn Andersson2, Jakob Öden2, Rasmus Bokrantz2
1RaySearch Laboratories, Physics department, Stockholm, Sweden; 2RaySearch Laboratories, Research department, Stockholm, Sweden; 3RaySearch Laboratories, Research Department, Stockholm, Sweden
Show Affiliations
Hide Affiliations
Purpose or Objective
High LET at the distal edge of proton beams may cause increased biological dose to OARs close to the target, and it is common practice to avoid spots where the Bragg peak is located right before them. A potential benefit of proton arc therapy is the many degrees of freedom compared to IMPT using only a few beams, thus increasing the possibility to remove high LET in OARs while still maintaining robust target coverage. This study investigates how LET-based robust optimization can improve IMPT and arc plans for a brain case.
Material and Methods
A research version of RayStation 12A contains the functionality to include robust optimization functions on the min/max dose-averaged LET (LETd), evaluated above a user defined dose threshold. It is also capable of planning for dynamic proton arcs, where the dose is delivered while the gantry is moving.
RayStation has been used to create a two-field IMPT plan and a dynamic arc plan for a brain case. Both were planned with identical robust optimization functions, assuming 3 mm setup and 3% range uncertainty: uniform CTV coverage of 54 Gy(RBE), max dose of chiasm and brainstem of 54 Gy(RBE) and a general reduction of dose in surrounding tissue. The plans were subsequently reoptimized with additional robust max LETd objectives of 3 keV/µm (above 5 Gy(RBE)) on the chiasm and brainstem. A robust evaluation (3 mm setup and 3% range uncertainty) was performed for all plans.
Results
Figure 1 shows the dose, LETd and DVHs in the nominal scenario. All plans have similar target coverage, and the near max dose to the OARs are below clinical thresholds (chiasm D2: 54 Gy(RBE), brainstem D2: 60 Gy(RBE)). High LET is clearly removed from the chiasm and brainstem in the LET optimized plans. This has been achieved by redistributing weight from spots with protons stopping before the OARs to spots from other angles, which can be seen in the LET-optimized IMPT plan where the posterior beam has a higher entrance dose. For the LET-optimized arc plan, spots passing through the brainstem get an increased weight, resulting in a higher low dose.
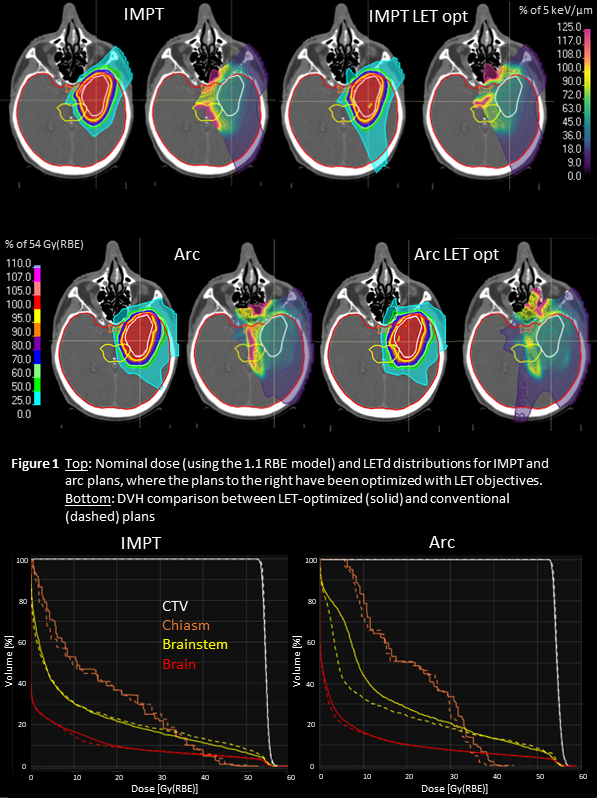
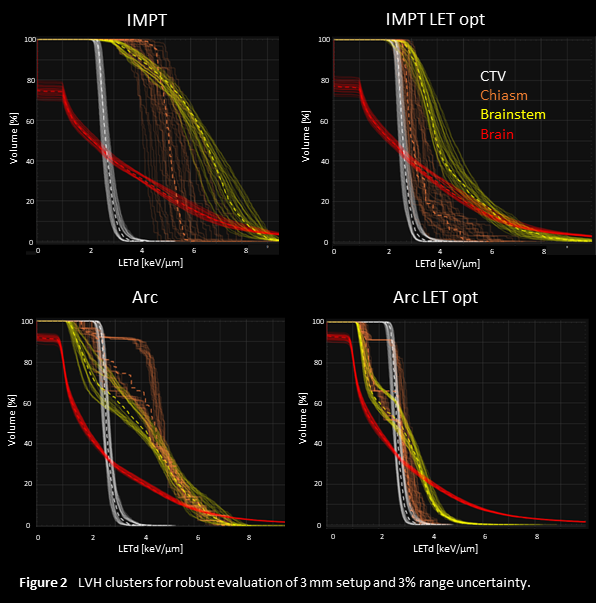
The robust evaluation of LETd is shown in Figure 2. Clinical goals with respect to dose were fulfilled for all plans in all scenarios, but there are significant differences in the LET volume histogram (LVH) clusters over all error scenarios. Both the mean and max LETd of the OARs are clearly reduced in the LET-optimized plans. The arc plans have lower LETd compared to the corresponding IMPT plans and show less variation over the error scenarios.
Conclusion
Combined dose and LET optimization can reduce high LET in OARs while maintaining robust target coverage. Compared to IMPT plans with a limited number of beams, dynamic arc plans are more suitable for reducing the LET in OARs, since multiple beam angles can be used to avoid protons stopping before OARs. The reduction of high LET in certain organs will affect the dose and LET in other regions, and the clinical impact of this should be analyzed carefully.