Single-institution clinical experience using robust IMPT in skull base chordoma and chondrosarcoma
Vesna Miladinovic,
The Netherlands
PO-1158
Abstract
Single-institution clinical experience using robust IMPT in skull base chordoma and chondrosarcoma
Authors: Vesna Miladinovic1,2, Yvonne Klaver1,2, Stijn Krol1,2, Michiel Kroesen2, Berit Verbist3,4, Steven Habraken5,2, Ida Coremans1,2
1Leiden University Medical Center, Radiation Oncology, Leiden, The Netherlands; 2HollandPTC, Radiotherapy, Delft, The Netherlands; 3Leiden University Medical Center, Radiology, Leiden, The Netherlands; 4HollandPTC, Radiology, Delft, The Netherlands; 5Erasmus MC Cancer Institute, Radiation Oncology, Rotterdam, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Chordomas and chondrosarcomas of the skull base are rare, slowly growing malignant bone neoplasms. Despite their radioresistant properties, proton therapy has been successfully used as an adjunct to resection or as a definitive treatment. In this study, we present the clinical results of robustly optimized intensity modulated proton therapy (IMPT) of twelve skull base chordoma and chondrosarcoma patients treated in HollandPTC, Delft, The Netherlands.
Material and Methods
We retrospectively reviewed clinical data, treatment details, and acute toxicity of all patients with chordomas and chondrosarcomas of the skull base treated with adjuvant IMPT between July 2019 and August 2021 in our institute. CT and 3.0T MR imaging examinations for treatment planning were performed in supine position in a thermoplastic mold. Robustly optimized proton therapy (PT) dose planning was performed with RayStation software (version 10B, RaySearch Laboratories, Stockholm, Sweden) and delivered with the Varian Probeam pencil beam scanning proton system (Varian Medical Systems, Palo Alto, CA, USA). A cumulative dose of 70-74Gy (RBE) was administrated to the CTV with 3 or 4 beams, in 35-37 fractions. Acute toxicity was scored weekly during the treatment, at the end of the treatment, and 3 months after the end of the treatment according to the CTCAE v4.0 scale.
Results
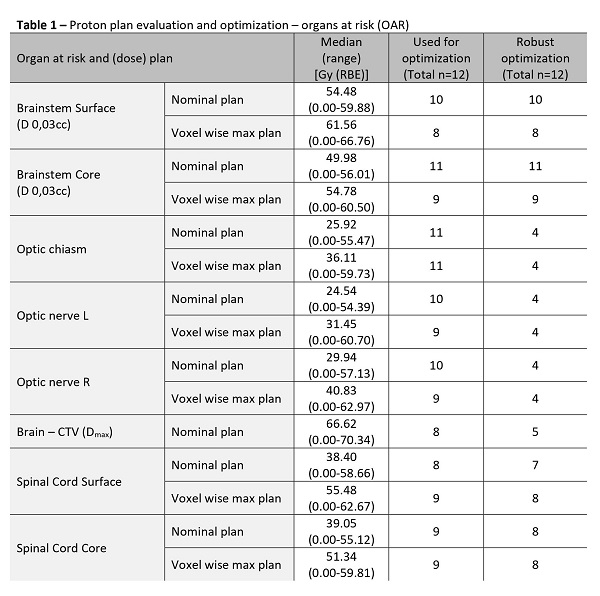
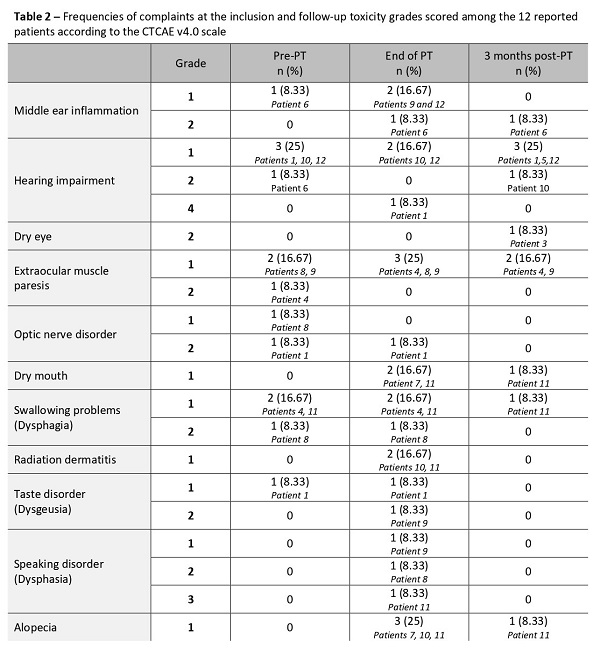
Four male and 8 female patients, 9 with chordoma and 3 with chondrosarcoma of the skull base were included. Nominal coverage of the high dose CTV was 97.83%, and 95.2% in the voxel wise minimum dose distribution. Plan optimization was mostly performed for the brainstem surface and core, and for the spinal cord surface and core. (Table 1) Evaluation CT scans during the treatment were made in eleven patients; nine patients had more than 3 evaluation CT scans, one patient had 2, and one patient had 1 evaluation CT scan. A total of 4 plan adaptations were made. Middle ear inflammation, dry mouth, radiation dermatitis, taste disorder, speaking disorder, and/or alopecia of grades 1-3 were noted at the end of the treatment among 6 patients without similar complaints at the inclusion. (Tabel 2) However, these symptoms disappeared 3 months following the treatment. Only for one patient dry mouth and alopecia complaints were still present 3 months after the treatment. One patient developed a temporary grade 4 hearing impairment at the end of treatment. Three months following the treatment one patient developed grade 1 hearing impairment and one patient developed grade 2 dry eye.
Conclusion
Robustly optimized IMPT is clinically feasible as an adjuvant treatment for skull base chordoma and chondrosarcoma patients. We observed acceptable early toxicities (grade 1-3) that tend to disappear in the first 3 months after irradiation.