Spinal ependymoma: Is treatment with proton therapy superior to photon therapy?
Shermaine Pan,
United Kingdom
PO-1150
Abstract
Spinal ependymoma: Is treatment with proton therapy superior to photon therapy?
Authors: Durga Harika Kannikanti1, Anna France2, Shermaine Pan1, Gillian Whitfield1
1The Christie NHS Foundation Trust, Networked Services- Proton beam therapy, Manchester, United Kingdom; 2The Christie NHS Foundation Trust, Proton Clinical Outcomes Unit, Manchester, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
Ependymomas constitute around 20% of all primary spinal cord tumours. Surgery may be followed by post-operative radiotherapy (RT) depending on extent of resection, histopathology and tumour grade. We retrospectively compared acute toxicity with post-operative proton and photon RT.
Material and Methods
We included all patients receiving proton RT at the Christie Hospital from 2018-2021 and all adults receiving photon RT from 2013-2018. For analysis, to account for 3 photon plans being 2-phase (initial larger volume and smaller boost volume), as well as differences in proportion of spine irradiated, we calculated Averaged Dose (Gy) = D1+(PTV2/PTV1)D2, and Normalised Dose = Averaged Dose × (PTV1/SL), where D1=phase1 dose (Gy), D2=phase2 dose (Gy), SL=spine length (cm), and PTV1 and PTV2 are the length (cm) of the Planning Target Volume (PTV) in phase1 and 2, respectively.
We collected acute RT toxicity data including haematological toxicity from patient notes. Linear regression models examined the effect of RT type on blood parameter nadirs (%), with a significance level of 5%. Box plots examined incidence of toxicities by RT type.
Results
Of 12 patients, 6 were female, and 6 treated with protons. Median ages were 42 years for photon RT and 13 years for proton RT. The Total Dose (D1+D2) for proton plans ranged from 50.4Gy to 54Gy in 28 to 30 fractions, and for photon plans 45Gy to 50.4Gy in 25 to 30 fractions (including phase2 doses from 9Gy to 15Gy in 5 to 10 fractions in 3 cases) (Table 1). The proton group had reduced incidence of alopecia, dysgeusia, mucositis, and nausea, but higher incidence of skin reaction and fatigue (Figure 1). The proton group maintained a significantly higher lymphocyte count (p = 0.029) (Figure 2). There was a trend to better maintenance of haemoglobin in the proton group, and of neutrophils in the photon group, neither statistically significant. There was no effect of RT type for platelet nadir.
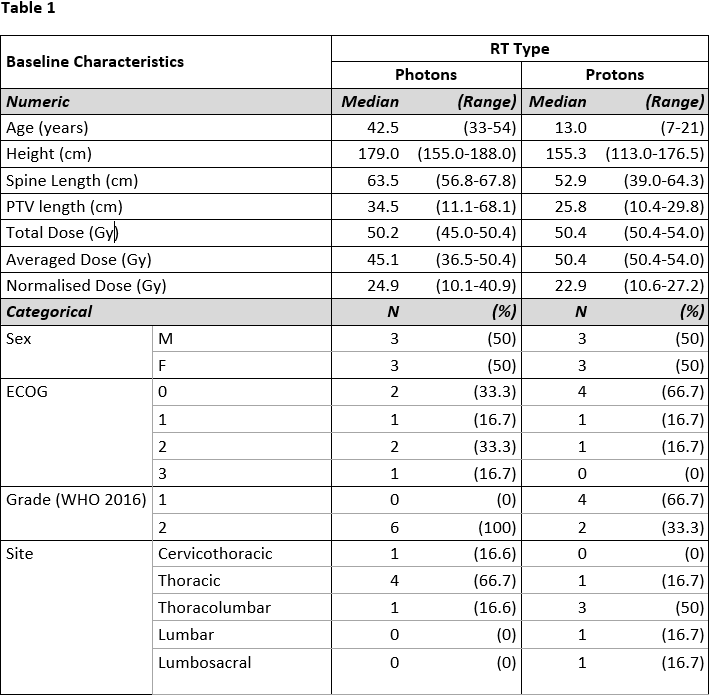
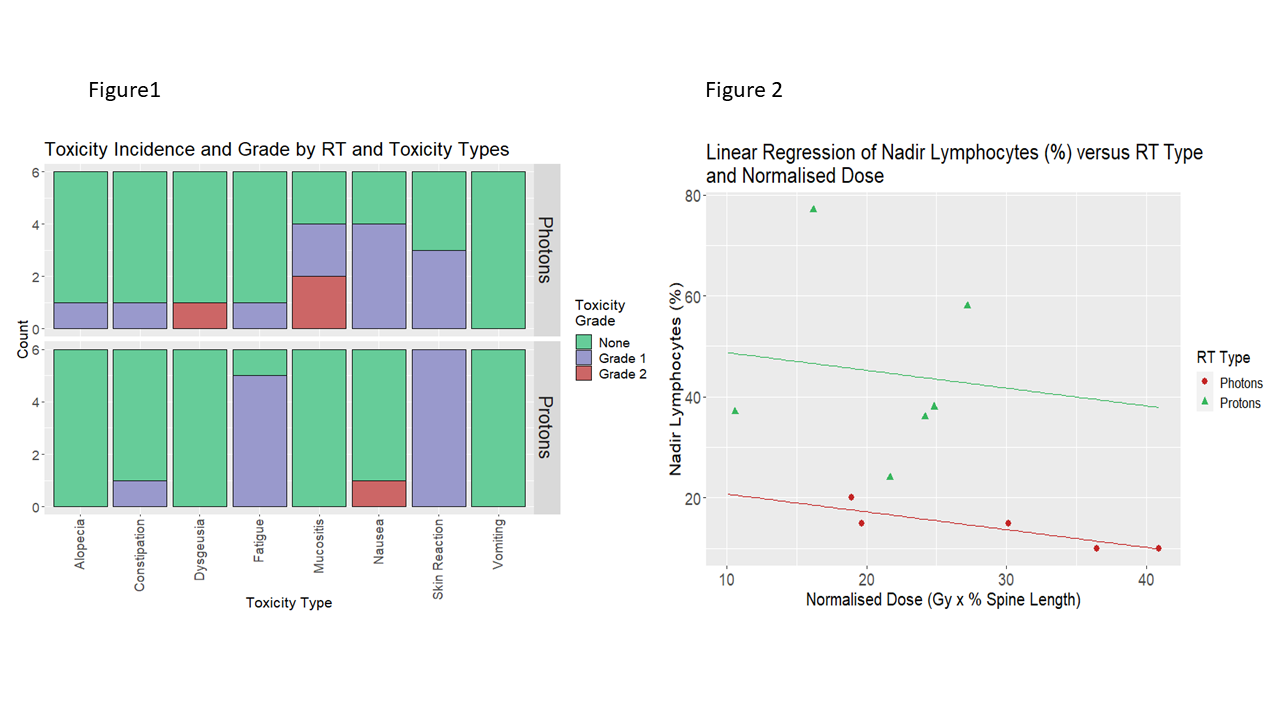
Conclusion
This small study illustrates a typical acute toxicity profile and suggests some limited differences in toxicity between proton and photon groups. Study limitations include the retrospective nature and different age profile of the two groups. Further data from larger patient series with detailed toxicity reporting would be needed to justify proton therapy for reduction in acute toxicity.