Safety and efficacy of silibinin in patients with brain metastases after SRS: SUSTAIN trial
PO-1109
Abstract
Safety and efficacy of silibinin in patients with brain metastases after SRS: SUSTAIN trial
Authors: Maria Grazia Carnevale1, Andrea Romei1, Ilaria Bonaparte1, Ilaria Morelli1, Marco Banini1, Isacco Desideri1, Viola Salvestrini2, Luca Visani3, Beatrice Detti1, Daniela Greto1, Lorenzo Livi1
1University of Florence, Radiation Oncology Unit, Azienda Ospedaliera Universitaria Careggi, Florence, Italy; 2Istituto Fiorentino di Cura ed Assistenza (IFCA), CyberKnife Center, Florence, Italy; 3stituto Fiorentino di Cura ed Assistenza (IFCA), CyberKnife Center, Florence, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
Brain metastases (BMs) account for more than one-half of all intracranial tumors. Over the past years, stereotactic radiosurgery (SRS) has become the mainstay of BMs treatment, providing high rates of local tumor control. Nevertheless, SRS alone is associated with a 30-50% risk of distant brain failure (DBF), consequently requiring careful follow-up and possible rescue treatments. Silibinin or silybin, a natural polyphenolic flavonoid, has shown promising antitumor activity, leading to improvement of BMs in patients (pts) with progressive non-small cell lung cancer. Therefore, our exploratory study aims to evaluate whether the use of a silibinin-based nutraceutical (SILLBRAIN®, HEALTH4U S.r.l.) can significantly reduce DBF rate at 6 months in pts with first-diagnosed BMs treated with SRS with or without surgery.
Material and Methods
SUSTAIN is an interventional, prospective, single arm, phase II study. A total of 80 pts treated in our center are planned to be enrolled. Pts receive 2 capsules (cps) of SILLBRAIN® per day for the first month after SRS and 1 cp per day thereafter. Primary endpoints are 6-month distant brain failure (DBF) rate and safety; secondary endpoint is 6-month overall survival (OS) rate. Contrast-enhanced magnetic resonance (MRI) of the brain is performed at baseline and every 12 weeks after SRS treatment; radiological response is assessed according to RANO criteria for brain metastases (RANO-BM)
Results
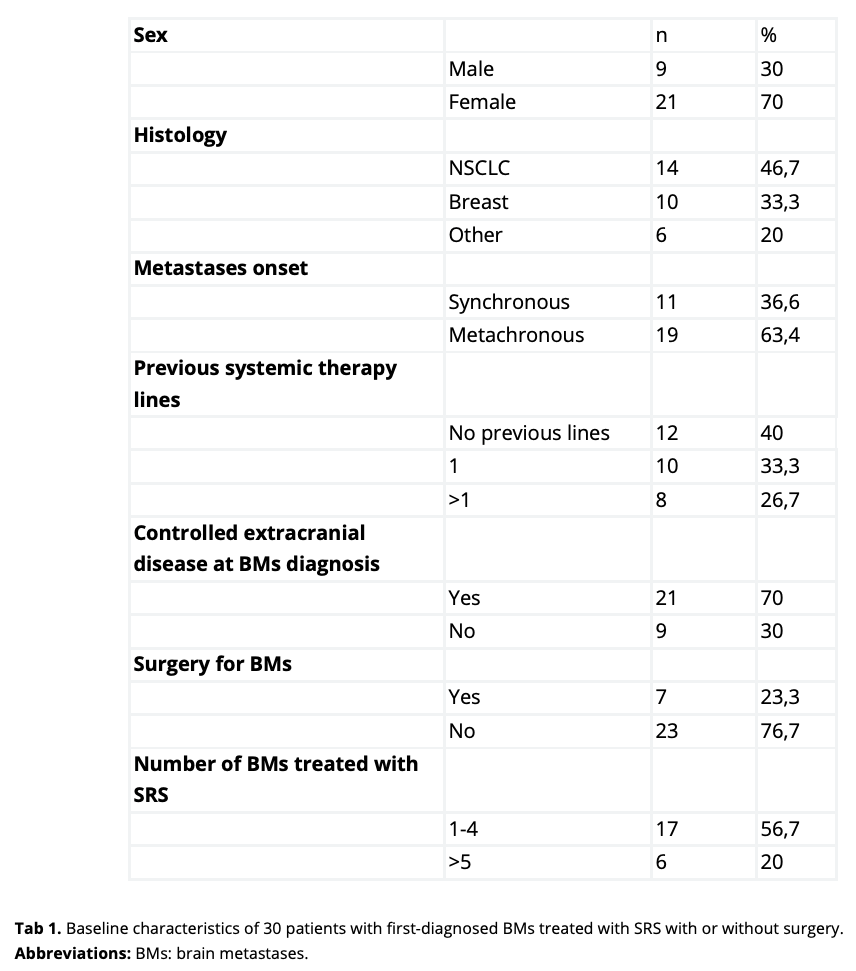
Thirty pts has been enrolled at the time of this primary analysis (Tab1). NSCLC and breast cancer were the prevalent histologies (14 and 10 cases respectively). Twelve pts were chemotherapy naive at the time of BMs diagnosis; 10 pts has received 1 systemic therapy line and 8 pts has received 2 lines or more. Overall, the number of treated lesions was 73, with a median of 1 lesion (range 1-9). All pts underwent SRS, with a median prescription dose (PD) and a median prescription isodose line (IDL) of 24 Gy and 80% respectively. After a 14-month enrollment period, with a median follow-up of 9 months, 6 pts reported distant intracranial failure according to RANO-BM criteria, with a 6-month DBF rate of 20%. Only 1 treated lesion met the criteria for progression, accounting for a 6-month local control (LC) rate of 98%. Six pts died, with a 6-month OS rate of 80%. Radiological radionecrosis was detected in 3 pts (10%), who developed G1 neurological symptoms managed with low-dose dexamethasone. Two pts discontinued SILLBRAIN® assumption due to G1 nausea. No other adverse events were reported.
Conclusion
At about half of our enrollment target, the results show a favorable trend towards 6-month DBF rate reduction. Toxicity proved manageable, consistent with the literature and limited to gastrointestinal effects. A larger sample and longer follow-up is needed to confirm our hypothesis.