SCOPE1 RTTQA - assessing adherence to tumour volume delineation guidelines
Rhiannon Evans,
United Kingdom
PO-2352
Abstract
SCOPE1 RTTQA - assessing adherence to tumour volume delineation guidelines
Authors: Rhiannon Evans1, Samantha Cox1, Tom Crosby1, John Staffurth1, Sarah Gwynne2
1Velindre Cancer Centre, Clinical Oncology, Cardiff, United Kingdom; 2South West Wales Cancer Centre, Clinical Oncology, Swansea, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
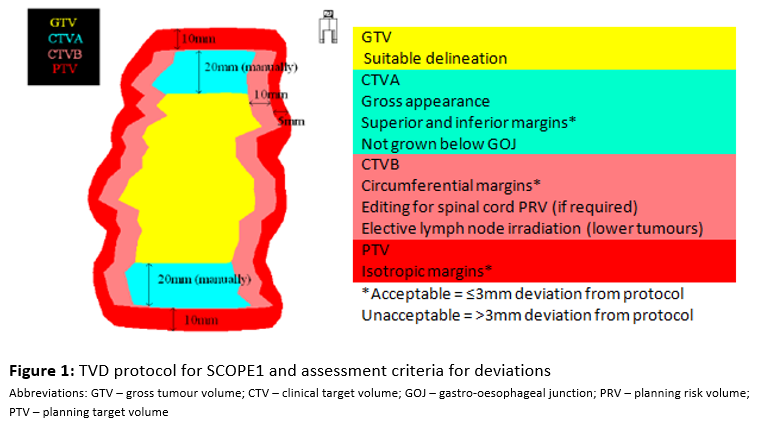
SCOPE1 was a phase 2/3 multi-centre trial of definitive chemoradiotherapy (dCRT) with Cisplatin and 5FU +/- Cetuximab for non-metastatic oesophageal cancer¹. In 2008, it standardised RT planning for this group of patients, providing the first consensus protocol for tumour volume delineation (figure 1) & a detailed QA programme. There was a pre-accrual QA programme with a benchmark middle third case. No benchmark case or worked examples of lower third cases were provided, especially with regard to elective lymph node irradiation (ELNI), which we now know is subject to interobserver variability. Given the now well established relationship between protocol adherence and outcome, we have subsequently undertaken retrospective individual case reviews of on-trial patients, with initial results presented at ESTRO in 2019²; here we present updated results.
Material and Methods
Only patients in the standard arm (no Cetuximab) were included for analysis, due to the trial showing addition of Cetuximab was detrimental. Using the Global Harmonisation Group’s (GHG) system for classifying RT protocol adherence, each step (protocol item) of TVD was retrospectively assessed. Cases without variations were graded as ‘acceptable-per protocol’. Variations in TVD which were unlikely to affect the trial outcome were graded as ‘acceptable’ (AV), whilst those with a potential impact were graded as ‘unacceptable’ (UV). UVs were further categorised according to their potential impact on tumour control probability (TCP) and toxicity. All cases were reviewed by a single RTQA reviewer, and 20% of plans underwent a secondary review by the RTQA lead for the SCOPE2 trial.
Results
91 RT cases were reviewed, with 16 protocol items for each (total 1456 items). 23 (25.3%) cases had no variations, and a further 25 (27.5%) were considered to have AVs from protocol. Whilst 43 (47.3%) cases had UV the majority of these only had 1 UV item, and therefore the overall percentage of UVs was small (5.8%). 2.8% of all UVs were considered to have potential impact on TCP, whilst 2.5% had potential to increase toxicity.
28/37 (75.7%) lower third oesophageal cases had UV’s compared to 15/54 (27.8%) upper/middle third cases. The difference is thought to be secondary to the complexity of ELNI, which was not assessed in the pre-trial benchmark case.
Conclusion
SCOPE 1, as the first multi-centre trial of dCRT in oesophageal cancer, aimed to standardise a TVD protocol. We have shown that the rate of UVs from protocol was less than 6%, which is encouraging, but leaves room for improvement. We have shown that components of a TVD protocol that are more complex and require more individual interpretation, such as ELNI, are the greatest source of variation. As trials in dCRT in oesophageal cancer increase in complexity with time, this study supports the need for an accompanying RTQA programme to minimise protocol variations as a confounder of outcome.