clinical outcomes of using 3D-printed applicators for HDR brachytherapy in gynecological malignancy
Wiwatchai Sittiwong,
Thailand
PO-2151
Abstract
clinical outcomes of using 3D-printed applicators for HDR brachytherapy in gynecological malignancy
Authors: Wiwatchai Sittiwong1, Pittaya Dankulchai1, Utumporn Puangragsa1
1Siriraj hospital, Radiology, Bangkok, Thailand
Show Affiliations
Hide Affiliations
Purpose or Objective
Brachytherapy plays an important role as the mainstay of treatment in several gynecological malignancies and image guided adaptive brachytherapy (IGABT) is considered the standard of care. The use of appropriate applicators is the key to achieve an adequate dose coverage to high-risk clinical target volume (HR-CTV). However, some patients cannot be applied with standard applicators due to the anatomy or tumor especially patients with recurrent tumor. 3D printing technology was adopted in high-dose-rate (HDR) brachytherapy treatment to create customized applicators for those patients in Siriraj hospital since 2021. This study was aimed to investigate the clinical outcome and toxicity in gynecological cancer patients treated with IGABT by using 3D-printed applicators.
Material and Methods
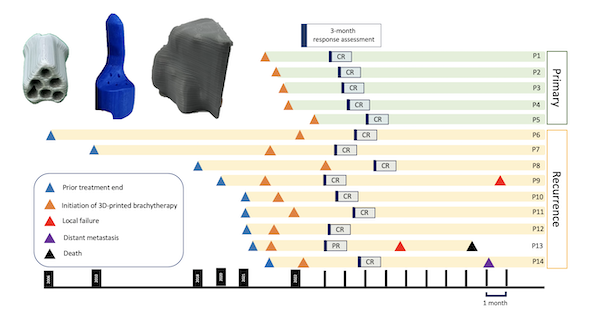
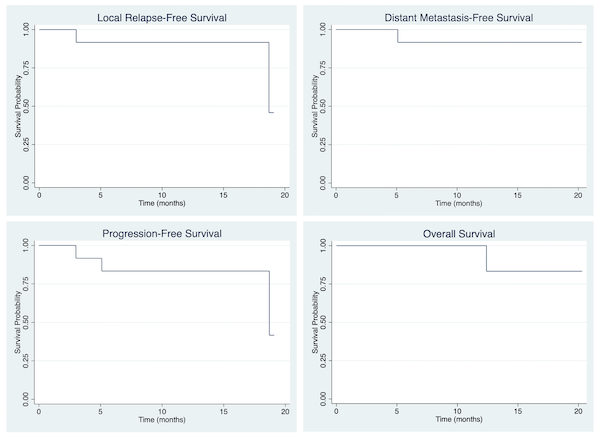
14 patients with gynecological cancer, both primary and recurrent patients treated with in-house 3D-printed applicators during 2021-2022 were retrospectively reviewed. Staging for each malignancy was based on FIGO 2018. Timing and pattern of response after treatment was reported and Kaplan-Meier estimates at 1 years were analyzed for relapse-free survival, distant metastasis-free survival, progression-free survival, and overall survival. The actuarial rates of acute and late genitourinary (GU), gastrointestinal (GI), skin and mucosa toxicity were reported.
Results
The median follow-up time was 11.4 months. 5 patients (35.7%) and 9 patients (64.3%) were categorized as primary and recurrent disease, respectively. For primary cases, 2 patients were cervical cancer, and 3 patients were extramammary Paget’s disease of vulva. For recurrent cases, all patients were vaginal relapse, and the most common primary cancers were cervical cancer (44.4%), and ovarian cancer (33.3%). The median time to 3D-printed applicator brachytherapy for recurrent cases was 23.2 months. The mean dose of D90 HR-CTV for patients received external beam radiation followed by brachytherapy and brachytherapy alone was 85.3 Gy (EQD2) and 49.3 Gy (EQD2), respectively. At 3 months after treatment, 13 patients (92.9%) achieve complete response (CR) and 1 patient (7.1%) was considered partial response (PR). 1-year local relapse-free survival, distant metastasis-free survival, progression-free survival, and overall survival were 91.7%, 91.7%, 83.3% and 83.3%, respectively. The actuarial rate of grade 2 acute GU and GI toxicity were 7.1% for both. For late GU and GI toxicity, the actuarial rates were 7.1% and 14.3%, respectively. For skin and mucosa toxicity, there were 4 patients (28.6%) developed acute grade 3 toxicity, and 1 patient (7.1%) had persistent grade 3 vaginal toxicity.
Conclusion
The treatment of IGABT by using 3D-printed applicators for HDR brachytherapy in difficult cases due to incompatibility of the applicators with patient’s anatomy or tumor demonstrated excellent outcomes and acceptable toxicity profiles.