MRI compatible tandem design for treatment of vaginal, cervical and endometrial brachytherapy
PO-2133
Abstract
MRI compatible tandem design for treatment of vaginal, cervical and endometrial brachytherapy
Authors: Akbar Beiki-ardakani1, Ivan Yeung2, Anna Simeonov2, Alexandra Rink2, Jette Borg2, Robert Weersink2, Michael Milosevic3, John Jezioranski2
1Princess Margaret Cancer Center, Radiation Physics, Toronto, Canada; 2Princess Margaret Cancer Centre, Radiation Physics, Toronto, Canada; 3Princess Margaret Cancer Centre, Radiation Oncology, Toronto, Canada
Show Affiliations
Hide Affiliations
Purpose or Objective
MR-compatible applicators composed of plastic or titanium are available commercially for gynecological brachytherapy (BT). All current commercial tandems are closed end, can only be used for cervical cancer with an existing canal and cannot be used without available Ring or Venezia systems. Further, commercial tandems are prone to breaking in the patient during overnight stay. To overcome these issues, we introduce a biocompatible open and closed-ended brachytherapy tandem for the treatment of endometrial, cervical or vaginal cancer.
Material and Methods
We designed and manufactured biocompatible polycarbonate tandems where dimensions and properties are similar to currently available designs, using a material that produces optimal MR images for BT planning when used with an in-house gel marker and kinks rather than cracks when first yielding to flexion (Figure 1a). Biocompatibility, sterilization, failure modes and material degradation were considered in the design and manufacture of the tandem. This device was designed to use either alone or together with commercially available applicators like the Best Medical® Syed Neblett template system. The tandems can be made either close or open-ended. A close-end version of our tandem has been used in 260 gynecological patients using HDR and PDR BT from 2003 to 2021. An open-end version, which designed for use with a needle and flexible obturator, was developed for vaginal, cervical, and endometrial cases.
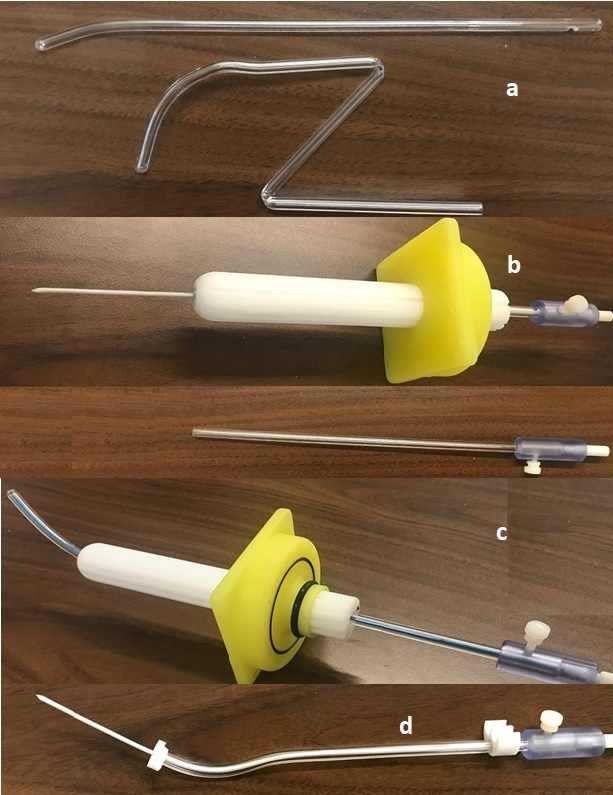
Figure1: a) Tandem durability due to kinks. b) Open end tandem used with template.
c) Curved tandem used with template. d) special open-end tandem for Ovid's.
Results
136 vaginal, cervical, and endometrial patients have been treated with our open or close-ended tandems in combination with Syed-Neblett template-cylinder system (Figure1b and 1c) since 2017 with decision made by our oncologists to use the in-house tandem rather than other commercial applicators. Average D90% (∝/b =10) for CTVHR was 90.0Gy± 4.7 Gy for 8Gy x 3 fractions or 7Gy x 4 fractions BT and 45Gy EBRT. In a single case study for bulky vaginal recurrence of endometrial cancer, CTVHR coverage improved from projected D90% =66.1Gy for the first insertion (8 Gy per fraction, Venezia with zero tandem and 6 needles) to 76.1Gy for the second insertion using the open-ended curved tandem (figure 1d) loaded with a needle in combination with the Venezia applicator and 7 needles). EQD2cc doses for sigmoid and bladder reduced from 70Gy and 83Gy to 61Gy and 75Gy, respectively.
Conclusion
We have introduced an alternative CT/MRI-compatible gynecological tandems that are safe, disposable, and have minimal imaging artifact. They are durable and withstands rigorous bending without breaking, minimizing the risk of fractured applicator and fragments left inside the patient. In summary, our open-end and closed-end tandems resulted in optimized brachytherapy plans for gynecological cancers when they used alone or in combination with other commercially available applicators.