Feasibility of clipless liver SABR: a new clinical online adaptive solution
PO-1904
Abstract
Feasibility of clipless liver SABR: a new clinical online adaptive solution
Authors: JULIEN PIERRARD1, Stéphanie Deheneffe2, David Dechambre3, Edmond Sterpin1, Tomasz Morgas4, Xavier Geets3,1, Geneviève Van Ooteghem3,1
1UCLouvain, Center of Molecular Imaging, Radiotherapy and Oncology (MIRO), Brussels, Belgium; 2CHU UCL Namur, Department of Radiation Oncology, Namur, Belgium; 3Cliniques Universitaires Saint-Luc, Department of Radiation Oncology, Brussels, Belgium; 4Varian Medical Systems, Varian Medical Systems Imaging Laboratory, Baden, Switzerland
Show Affiliations
Hide Affiliations
Purpose or Objective
Optimal IGRT in liver SABR requires invasive implantation of radio-opaque clips. We planned a retrospective in silico feasibility study of clipless liver SABR using the ETHOS® (Varian Medical Systems, Palo Alto, CA) linac allowing online adaptive radiotherapy (ART). Accuracy of GTV propagation and dosimetric impact were analysed.
Material and Methods
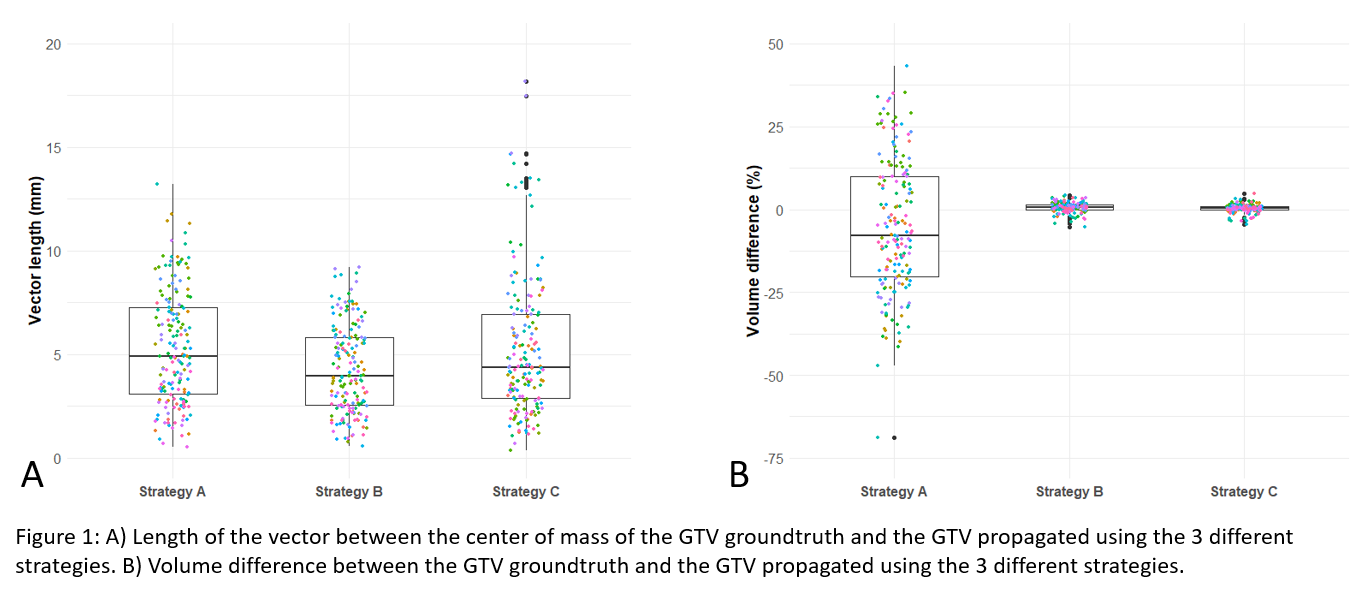
Simulated SABR adaptive treatment plans were made for 21 liver tumors surrounded by clips. Virtual RT sessions were delivered on daily CBCTs (n=174) using 3 GTV propagation strategies: (A) influencers-guided deformable GTV propagation; (B) Rigid GTV mapping based on image-guided deformable planning (p)CT-CBCT registration; (C) Rigid GTV mapping based on automatic rigid pCT-CBCT registration. The GTV propagated was compared with the GTV groundtruth (manual rigid pCT-CBCT registration based on clips followed by rigid GTV propagation). The GTV propagation accuracy was determined using 2 metrics : the vector length between centers of mass (VL) and the volume difference between GTVs (ΔV).
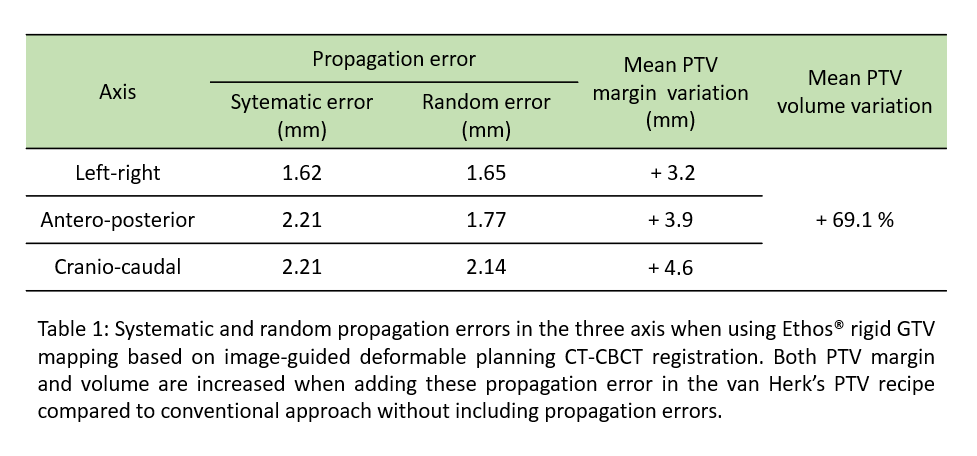
For the best strategy, the propagation error was computed and added in a modified PTV margin (mPTV) based on van Herk’s recipe and confirmed on 11 liver SABR with no clips (75 CBCTs). Dosimetric analysis was done for 20/32 patients of the whole cohort for which 4D-pCT were available. Comparison was made between conventional plans (Plan A) and ETHOS® ART plans (Plan B) (sum of daily adapted plans using the mPTV margin) for a dose of 5x10Gy. The dose constraints of organs at risk were reported and ranked in order of importance : priority 1 (n=17), priority 2 (n=1) and priority 3 (n=7). Target coverage objectives were also compared (Wilcoxon test).
Finally, the same analysis was performed for each ETHOS® session (n=96) to compare scheduled and daily adapted plans.
Results
GTV propagation accuracy : Mean VL was 5.3, 4.3, and 5.2mm and ΔV ranges were [-68.9;43.3]%, [-5.4;4.3]% and [-4.4;4.8]% for propagation strategies A, B and C respectively (Figure 1).
Dosimetric analysis : mPTV volume is increased by 69.1% in average compared to conventional PTV (Table 1). Dose constraints violations in plan A were observed in 0% (0/337) for priority 1, 5% (1/20) for priority 2 and 8.6% (12/140) for priority 3. In plan B, there were 2.7% (9/337) priority 1, 5% (1/20) priority 2 and 13.6% (19/140) priority 3 dose constraints violations. Mean GTV D95%, GTV D110% and PTV V100% were similarly respected for both plans (p>0.05).
Finally, there were 4.4% (74/1697) priority 1, 5.0% (5/100) priority 2 and 15.0% (105/700) priority 3 dose constraints violations when using scheduled plans and 0.8% (14/1697) priority 1, 6.0% (6/100) priority 2 and 12.7% (89/700) priority 3 violations when using daily adapted plans. Mean GTV D110% was higher for daily adapted plans (p<0.001), other coverage objectives were similar.
Conclusion
Clipless liver SABR is feasible on ETHOS® at the cost of increased PTV margins. Daily plan adaptation is required and allows to better respect the dose constraints.