Respiratory motion prediction based on LSTM and linear regression models
PO-1886
Abstract
Respiratory motion prediction based on LSTM and linear regression models
Authors: Lukas Wimmert1, Maximilian Nielsen1, Tobias Gauer2, Christian Hofmann3, Rene Werner1
1Computational Neuroscience, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; 2Radiotherapy and Radiation Oncology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; 3Siemens Healthcare, /, Erlangen, Germany
Show Affiliations
Hide Affiliations
Purpose or Objective
Available motion management systems account for tumor motion with approaches such as tracking or gating to reduce normal tissue dose. However, the systems have to compensate for latencies using respiratory motion predictors. Present motion predictor comparison studies work on denoised breathing signals with a fixed short-term prediction horizon (usually 400-500ms). To potentially avoid required additional latencies for signal denoising, we extend the studies to noisy input signals, considering state-of-the-art prediction approaches: linear regression (LR) and long-short term memory (LSTM). In addition, we analyze the performance for longer prediction horizons required for other 4D SBRT applications (eg, CT and CBCT gating).
Material and Methods
The study is based on 2517 respiratory signals (sampling rate 25Hz; length of signals between 30 and 300s) of 418 lung and liver SBRT patients, recorded with the Varian RPM system during planning 4D CT, pre-treatment 4D CBCT and gated dose delivery. Signals were randomly divided into training (number of patients n=215, number of total signals # 1265), validation (n=85, # 516) and test (n=118, # 736) set on patient level. Each signal was rescaled to an amplitude range [-1, 1], based on min/max scaling of its first 20s. To standardize noise conditions, each signal was Fourier-smoothed (1 Hz cutoff frequency), and white noise (SNR=27dB) was added afterwards. Inputs were sampled by an overlapping sliding window approach. Based on the noisy signal, the task was to predict the respiratory amplitude for the prediction horizon ahead (single output value prediction). Target signals were the denoised signal to avoid overfitting the models to the noise. The training loss was the mean squared error (MSE) between model output and the denoised target signal. Prediction horizons were 480ms (similar to literature), 760ms, and 1000ms.
Results
A representative case is shown in Figure 1. LR and LSTM test set prediction errors are summarized in Table 1. In line with current literature, the LR model performs almost perfectly on the denoised data and the short prediction horizon. However, for longer prediction horizons and noisy input, the prediction accuracy drops. Here, the LSTM model outperforms the LR model. For a 1s prediction horizon, the LSTM with noisy input performs on par with LR on denoised input data.
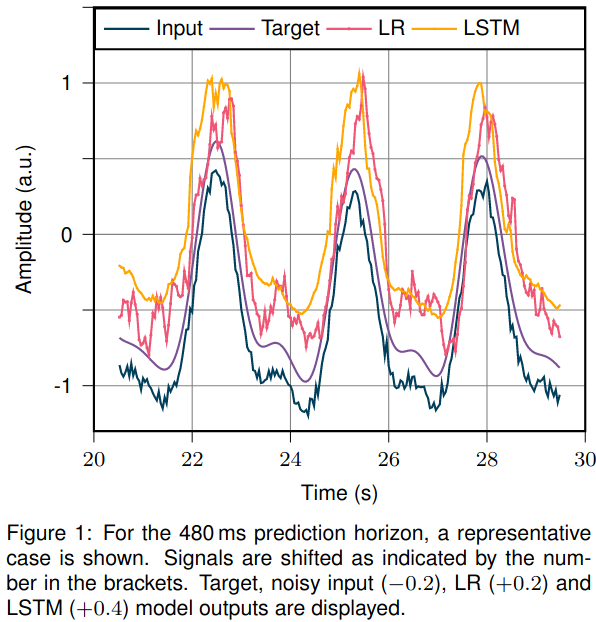
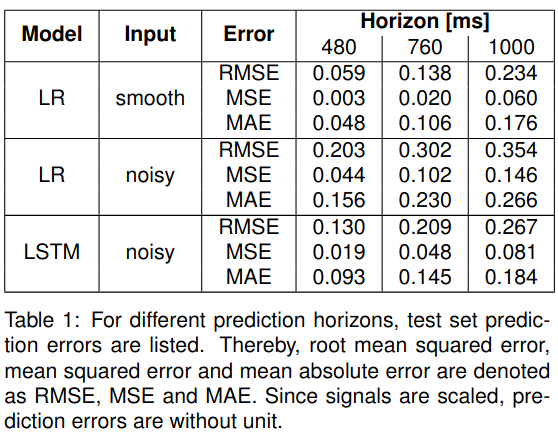
Conclusion
The results illustrate that with increasing complexity of the prediction task (longer prediction horizon, noisy data), deep learning (here: LSTM) outperforms the current state-of-the-art LR for respiratory signal prediction to overcome system latencies in SBRT of thoracic tumors.