Feasibility of in vivo diffusion weighted imaging on a 0.35 T MRI-guided linear accelerator
PO-1820
Abstract
Feasibility of in vivo diffusion weighted imaging on a 0.35 T MRI-guided linear accelerator
Authors: Joseph Weygand1, Tess Armstrong2, J.M. Bryant1, Jacqueline Andreozzi1, Ibrahim M. Oraiqat1, Casey L. Liveringhouse1, Kujtim Latifi1, Kosj Yamoah1, James R. Costello3, Eduardo G. Moros1, Issam M. El Naqa4, Arash O. Naghavi1, Stephen A. Rosenberg1, Gage Redler1
1Moffitt Cancer Center, Radiation Oncology, Tampa, USA; 2ViewRay, Product Development, Oakwood Village, USA; 3Moffitt Cancer Center, Radiology, Tampa, USA; 4Moffitt Cancer Center, Machine Learning, Tampa, USA
Show Affiliations
Hide Affiliations
Purpose or Objective
Diffusion weighted imaging (DWI) noninvasively interrogates tissue cellularity as a surrogate for cellular proliferation. Incorporation into the adaptive workflow on a 0.35 T MRI-guided linear accelerator (MRL) to produce apparent diffusion coefficient (ADC) maps facilitates identification of cellular subpopulations with restricted diffusion and enables dose escalation strategies and/or biologically-guided plan adaptation that could be clinically advantageous. However, DWI on the 0.35 T MRL has struggled due to technical challenges that have now been resolved. In this work, the accuracy, stability, and geometric precision of ADC maps produced using a novel echo planar imaging (EPI)-based DWI protocol on the MRL is demonstrated in phantom, and the potential for longitudinal patient imaging is illustrated in a sarcoma patient receiving same-day diagnostic DWI.
Material and Methods
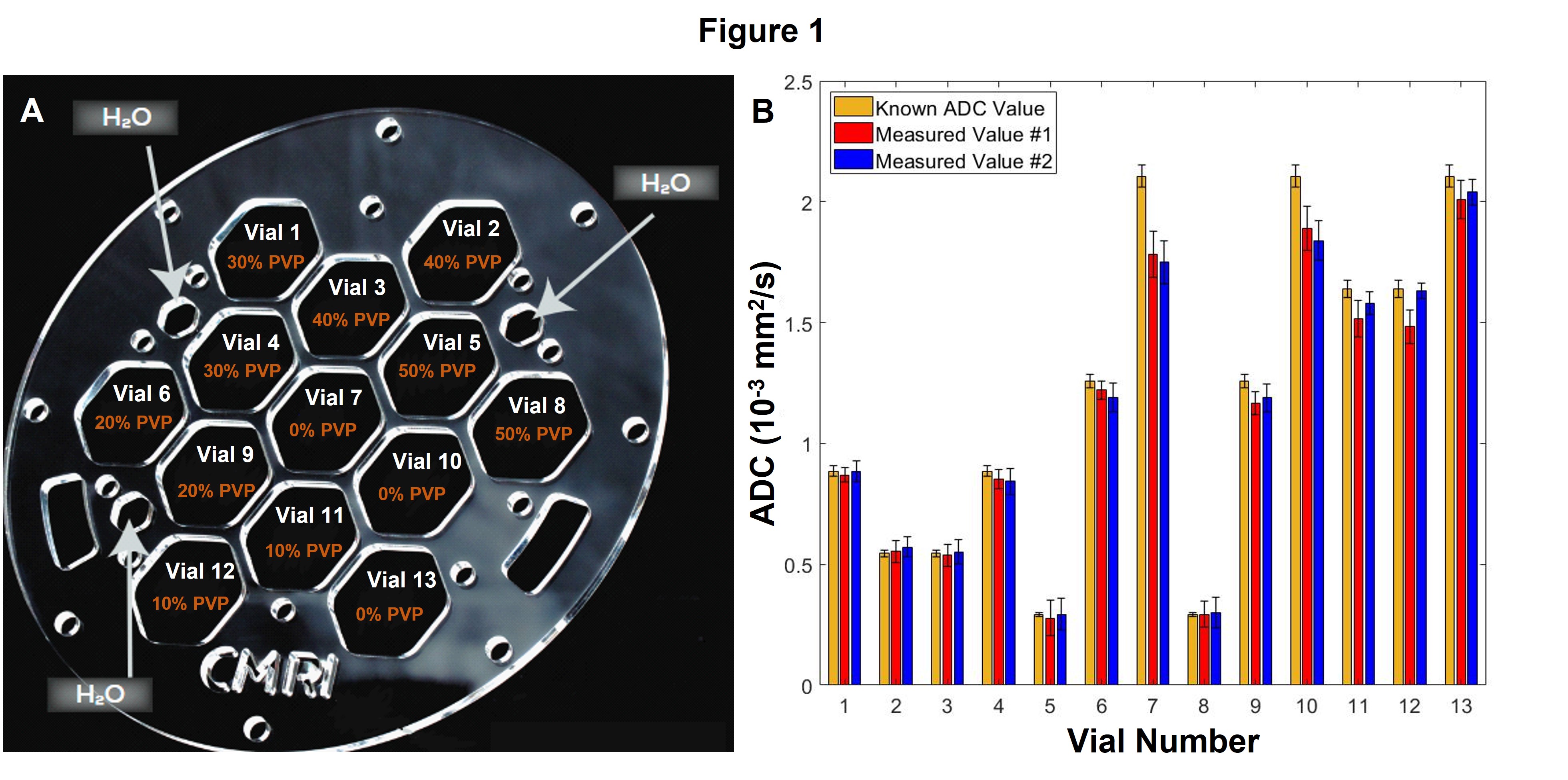
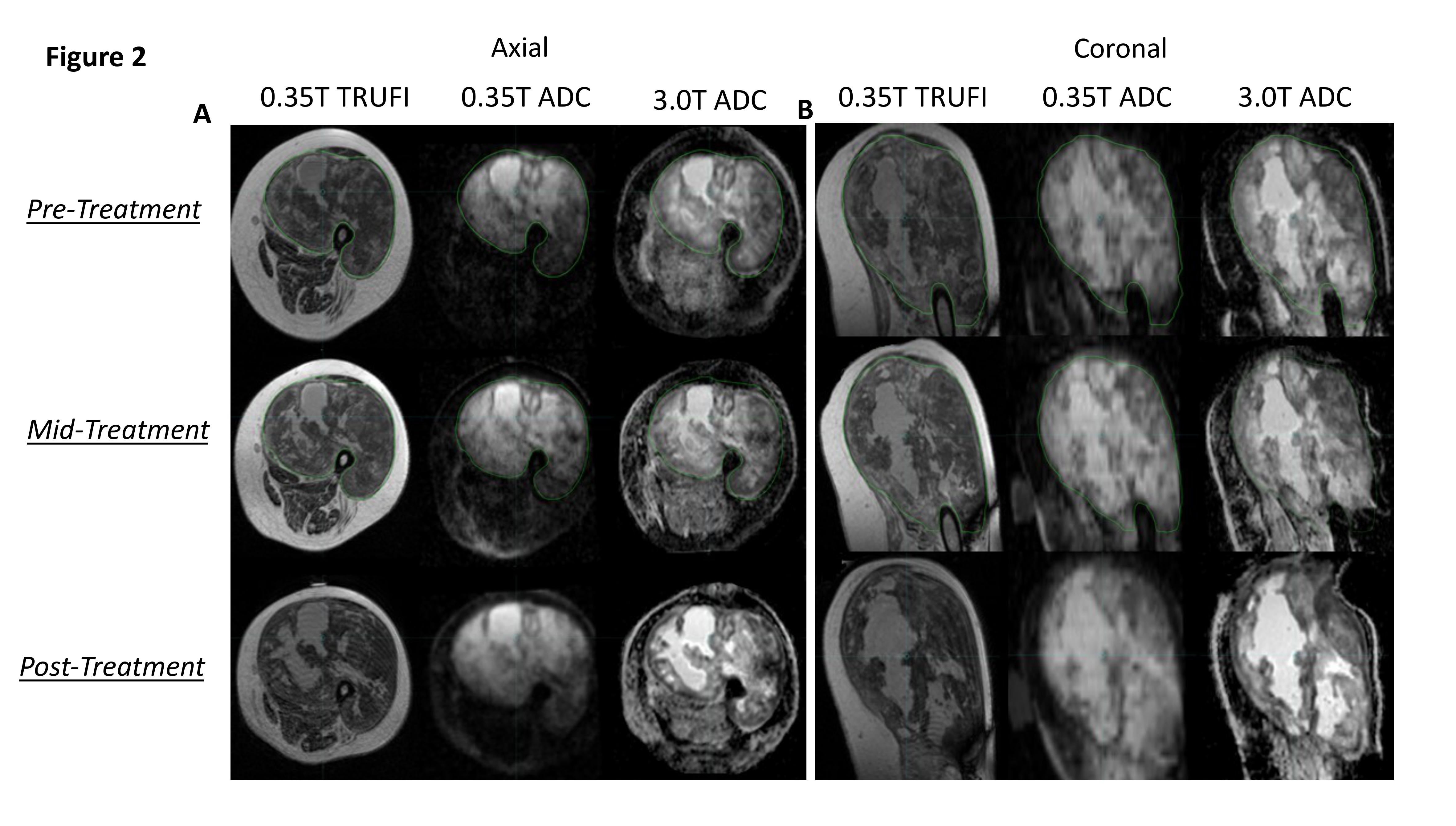
A NIST-traceable diffusion phantom with vials of known variable ADC was imaged. Accuracy and constancy were assessed by measuring ADC values at two timepoints and comparing to NIST-traceable ADC. System-dependent geometric distortion was quantified by measuring the distance between 93 pairs of phantom features in a central slice and a slice 5.4 cm off-center, for comparison to measurements from a spatially precise CT. This analysis was performed by three independent observers for ADC maps taken on the 0.35 T MRL and a 3 T diagnostic scanner. Additionally, for a sarcoma patient receiving radiotherapy on the MRL, same-day in vivo ADC maps were acquired on both systems at three timepoints: pre-(day 0), mid-(day 21), and 3 weeks post-(day 69) treatment.
Results
Phantom ADC quantification was accurate with discrepancies beyond measurement uncertainty only for vials approaching free diffusion. ADC quantification was constant over repeat measurements with small discrepancies only seen at high ADC (Figure 1). In the central slice, average geometric distortions were 0.35 (±0.02) mm and 0.85 (±0.02) mm for the MRL and diagnostic system, respectively. At 5.4 cm off-center, these were 0.66 (±0.04) mm and 2.14 (±0.07) mm for each, respectively. In the sarcoma patient, image quality and ADC features were comparable at each timepoint. Additionally, the region of enhanced ADC in MRL images demonstrates marked conformity to tumor contours delineated on anatomical (True FISP) images and similarity with tumor ADC features visualized in the diagnostic DWI (Figure 2).
Conclusion
The acquisition of accurate, reproducible, and geometrically precise (sub-millimeter distortion; >2x improvement over 3T) ADC maps is possible at 0.35 T with a novel EPI approach. This enables tracking of longitudinal changes in tumor cellular density during MRL treatment and will facilitate personalization/adaptation not only to patient anatomy but dynamic tumor physiology as well.
* Dr. Rosenberg and Dr. Redler contributed equally as senior authors.