Stereotactic Body Radiotherapy (SBRT) for Spinal Metastases
PO-1595
Abstract
Stereotactic Body Radiotherapy (SBRT) for Spinal Metastases
Authors: Federica Ferrario1,2, Miriam Torrisi1,2, Chiara Chissotti1,2, Laura Giannini1,2, Chiara Lucrezia Deantoni1, Sara Broggi3, Martina Midulla1,2, Stefano Lorenzo Villa1,2, Italo Dell'Oca1, Andrei Fodor1, Claudio Fiorino3, Antonella Del Vecchio3, Stefano Arcangeli4,2, Nadia Gisella Di Muzio1,5
1IRCCS San Raffaele Scientific Institute, Department of Radiation Oncology, Milan, Italy; 2Università degli Studi di Milano Bicocca, School of Radiation Oncology, Milan, Italy; 3IRCCS San Raffaele Scientific Institute, Medical Physics, Milan, Italy; 4ASST Monza, Department of Radiotherapy, Monza, Italy; 5Università Vita-Salute San Raffaele, Faculty of Medicine and Surgery, Milan, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
Spine is a common site of metastatic bone disease and represents a challenging treatment for radiation oncologists due to the presence of the spinal cord as close organ-at-risk.
The aim of this study is to report our experience with robotic SBRT for the treatment of spine metastases, evaluating different fractionation schedules, in terms of efficacy and toxicity.
Material and Methods
This retrospective study includes a total of 133 treatments in 79 patients (pts), delivered between October 2017 and November 2021, on spine metastases. Patient’s median age was 72 (21-91) years at the time of SBRT. Primary tumor was prostate in 45 pts, breast in 10 pts, lung in 8 pts, and other primary tumors in the remaining pts.
SBRT was delivered with CyberKnife® (Accuray, Sunnyvale, CA, USA) real-time tracking radiation therapy. Depending on the metastasis location, Clinical Target Volume (CTV) was defined as whole vertebral body +/- pedicles +/- posterior elements, according to the International Spine Radiosurgery Consortium consensus guidelines.
Different fractionation schedules were used and compared: single fraction (fx) for 90 treatments versus 3-5 fx for 43 treatments. In the single fx the median prescription dose was 18 (16-20) Gy, to the median isodose of 79 (70-83) %. In the fractionated treatments, median prescription dose was 27 (24-35) Gy, to the median isodose of 79 (70-82)%.
Forty-nine pts were treated with precautional steroid therapy at the time of RT.
Toxicity assessment was based on CTCAE version 5.0 criteria.
Results
Median follow-up was 37.8 (1.1-60.2) months.
No grade (G) ≥ 3 acute toxicity was observed. One patient suffered from G2 hyposthenia and paresthesia of the lower limb, three pts experienced G1 back pain, one patient had G1 nausea. No late toxicity was observed.
Overall Survival (OS) at 12- and 24- months was 84.7%, and 62.7%, respectively. Kaplan Meier estimates of local relapse free survival (LRFS) was 86.1% at 12 months, 77.5% at 24 months, 71.9% at 48 months and 67.4% at 60 months (See Fig. 1). Median distant metastases free survival (DMFS) was 9.3 months. A trend for a significant difference (p=0.06) in distant metastases-free survival (DMFS: 10.3 vs 8.4 months, respectively) in favor of single versus multiple SBRT fractions was observed (See Fig. 2). In the single fx group 47.8% of pts had up to four metastases, while in the 3-5 fx group 51.2% of pts had up to four metastases.
Before treatment 36 pts were symptomatic, and after SBRT 89% of them presented a complete pain response.
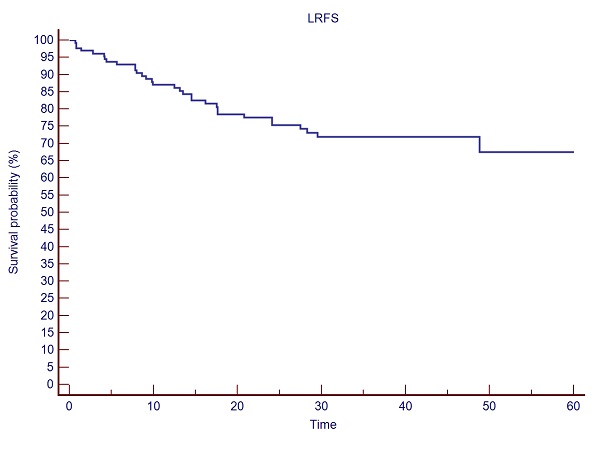
Fig. 1: LRFS after spine SBRT for bone metastases.
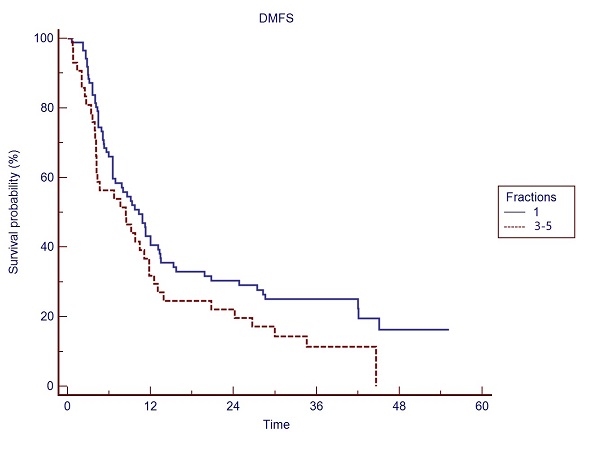
Fig. 2: DMFS in the single fx vs 3-5 fx treatments.
Conclusion
Spine SBRT appears clinically feasible and safe, allowing the delivery of ablative/radical doses to the target, while sparing the critical organs-at-risk, namely the critical neural tissues with good local control and low toxicity. In our experience, a trend to a better DMFS was obtained with a single fx vs 3-5 fx in the two fractionation groups, with similar disease burden.