two years of ocular proton therapy in The Netherlands, clinical results
PO-1587
Abstract
two years of ocular proton therapy in The Netherlands, clinical results
Authors: Coen Rasch1, P Bakker2, M Rodrigues2, M Marinkovic3, K Vu3, J Bleeker4, C van Rij5, E Kilic6, JW Beenakker7, N Horeweg8, G Luyten3
1LUMC, Radiation Oncology, Leiden, Nauru; 2Holland Proton Therapy Center, Radiotherapy, Delft, The Netherlands; 3Leiden University Medical Center, Ophthalmology, Leiden, The Netherlands; 4Leiden University medical Center, Ophthalmology, Leiden, The Netherlands; 5Erasmus Medical Center, Radiation Oncology, Rotterdam, The Netherlands; 6Erasmus Medical Center, Ophthalmology, Rotterdam, The Netherlands; 7Holland Proton Therapy Center, Radiology, Delft, The Netherlands; 8Leiden University Medical Center, Radiation Oncology, Leiden, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Uveal Melanoma is a rare cancer with a rising incidence of 143 in 1990 to 239 in 2019 in the Netherlands. Local treatment consists of brachytherapy, external beam radiotherapy (proton or photon) or enucleation. Most patients are treated locally with ruthenium brachytherapy (+/- 102 /year) but those with a tumor prominence of >7mm, and/or a basal diameter >16 mm and/or involvement of the optic disc (>⅓ of the circumference) are referred for proton therapy. Proton Therapy for Ocular Melanoma is available in The Netherlands from Jan 2020 onwards. The purpose of this abstract is to evaluate the first two-year results.
Material and Methods
All consecutive patients treated with proton therapy for Uveal Melanoma from Jan 2020 till December 31st 2021 were included in this evaluation (n=56). Pre-treatment imaging consists of ultrasound and 3Tesla MRI, followed by operative placement of tantalum clips on the sclera at the edge of the tumor and post-operative MRI. Proton treatment is performed with a dedicated passive scattered beam using an individual mold in the Eye Treatment Room at the Holland Particle Center (Delft, The Netherlands). The prescribed dose is 4x15 (Cobalt Equivalent) Gy on four consecutive days. Treatment planning is performed in Eclipse Ocular Proton Planning (EOPP, Varian Medical Systems, Palo Alto). Patients have regular FU in the referring center (LUMC or ErasmusMC). Time to event analyses were conducted according to the Kaplan-Meier method.
Results
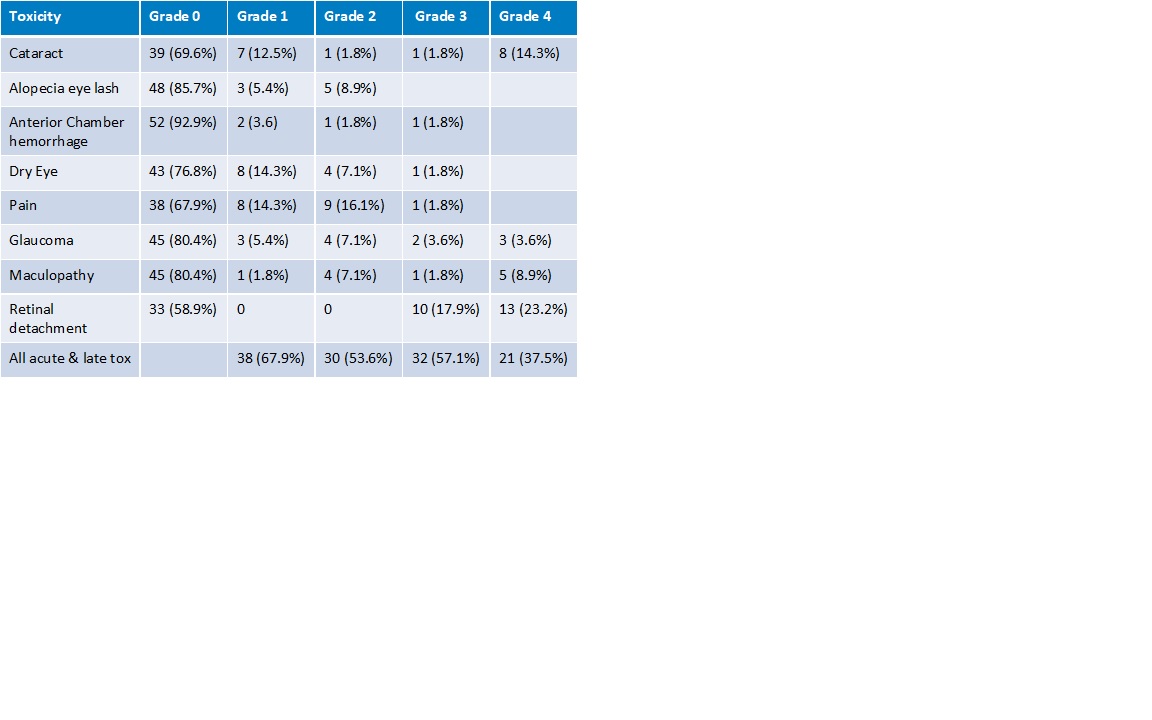
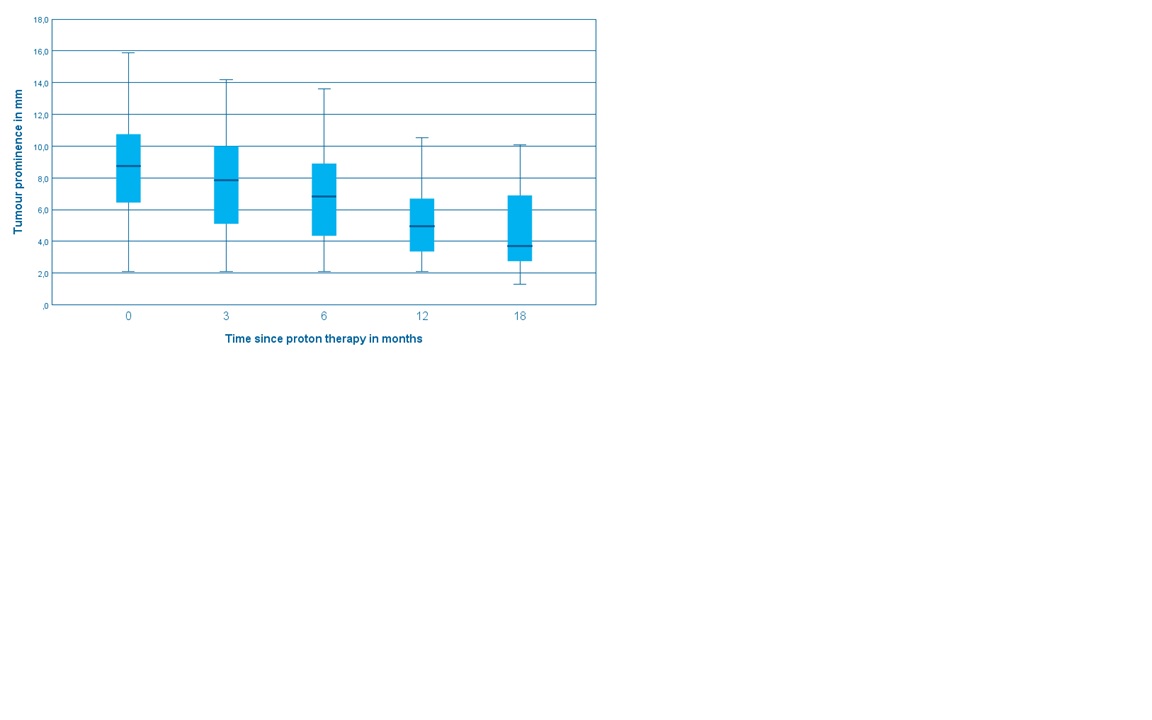
56 patients with a median FU of 1 year were included in this study. The median age was 64 years. The tumors were central 12 (21%), midperipheral 29 (52%) and peripheral 15 (27%). Nineteen (34%) had a juxtapupillary location. Tumor stage: T1 5 (9%), T2 12 (21%), T3 32 (57%), T4 7 (13%). Retinal detachment before treatment was present in 42 cases (75%). Tumor regression was slow and continued across the FU period (Fig.1). At two years, Overall Survival was 86% (T1-2 100%, T3-4 79%), DSS was 89%, and local recurrence-free survival was 89%. All patients with T1-2 tumors retained their eye, while eye preservation was 73% at two years for T3-4, mostly because of retinal detachment. Severe visual impairment was 58% at two years. 32 (57.1%) and 21 (37.5%) patients had Grade III and IV toxicity respectively (Table 1).
Conclusion
Proton Therapy for ocular melanoma in The Netherlands is the treatment of choice when brachytherapy treatment is not feasible anymore. The treatment results in ocular preservation in the majority of the cases.