SBRT for prostate cancer with or without Elective Nodal Irradiation
PO-1454
Abstract
SBRT for prostate cancer with or without Elective Nodal Irradiation
Authors: Juan Canales1, Tomás Merino1, Paula Reyes1
1Pontificia Universidad Católica de Chile, Hemato-oncology, Santiago, Chile
Show Affiliations
Hide Affiliations
Purpose or Objective
Feasibility to randomize and assess acute toxicity of patients with unfavorable intermediate-risk and high-risk to prostate and seminal vesicle with or without Elective Nodal Irradiation (ENI) with SBRT in Academic Center in Latin America. In addition, evaluate the fidelity of the treatment protocol and the dose restriction to the organ at risk (OAR).
Material and Methods
Patients with unfavorable intermediate and high risk prostate cancer were recruited. Randomization was to prostate and seminal vesicles alone (Arm 1) 36.25 Gy in 5 fractions versus the same treatment plus ENI at a dose of 25 Gy as with a simultaneous boost (Arm 2). The primary outcomes was Recruitment (Percentage of screened patients eligible recruited in six months) and Treatment protocol Fidelity, defined as protocol compliance or minor deviation for PTV/CTV and organ at risk (OAR) coverage. Recruitment success was defined as 80% and Treatment protocol Fidelity as 60%. The secondary outcome was acute genitourinary (GU) and gastrointestinal (GI) adverse events grade 3 or greater, according with the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03, 1 month after the end of radiotherapy.
Results
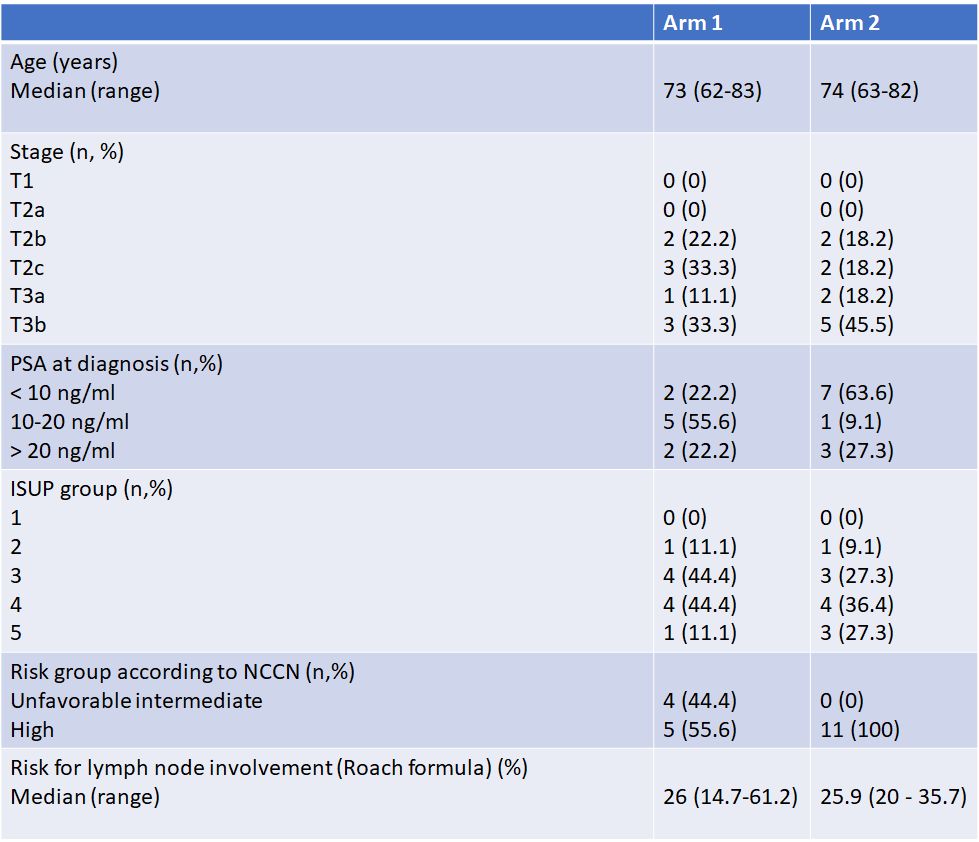
From Aug 2021 to Jan 2022, 30 patients met the inclusion criteria and of these four declined to participate. Eleven patients were randomized to Arm 1 and thirteen to Arm 2. Recruitment was 86.7%. Four patients did not receive allocated treatment due to intercurrent disease (2) or severe incontinence (2). Finally, nine and eleven patientes recived treatment to wich they were randomized. The characteristics of patients are summarized in Table 1. None and two patients report major deviations in PTV prostate+seminalvesicle coverage in Arm 1 and Arm 2, respectively; no major deviations in CTV prostate+seminalvesicle, PTV pelvis and CTV pelvis were found in Arm 2. Regarding OAR, 3 and 2 patients had major deviations on the rectum in Arm 1 and Arm 2, respectively. Treatment protocol fidelity for PTV/CTV was 100% and 81.8% in Arm1 and Arm2, respectively, and for OAR 66.7% and 81.8% in Arm 1 and Arm 2, respectively. At one month after the end of treatment one patient reported acute GI toxicity grade 3 (diarrhea) in Arm 2, without acute grade 3 or higher GU toxicity. No grade 3 or higher GI and GU was present in Arm1.
Conclusion
Previously defined recruitment and treatment fidelity standards were met. Conducting a randomized study in this scenario is feasible. Acute toxicity is low. Longer follow-up is needed to assess adherence and long-term toxicity and efficacy.