HERMES: Are we delivering?
Rosalyne Westley,
United Kingdom
PO-1501
Abstract
HERMES: Are we delivering?
Authors: Rosalyne Westley1,5, Alex Dunlop2, Adam Mitchell2, Stefanos Diamantopoulos2, Sophie Alexander3,4, Trina Herbert3, Jonathon Mohajer2, Uwe Oelfke2, Alison Tree5,1
1The Royal Marsden NHS Foundation Trust, Uro-oncology, Sutton, United Kingdom; 2Joint Department of Physics, The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust, Physics, Sutton, United Kingdom; 3The Royal Marsden NHS Foundation Trust, Radiotherapy, Sutton, United Kingdom; 4The Institute of Cancer Research, Radiotherapy, Sutton, United Kingdom; 5The Institute of Cancer Research, Uro-oncology, Sutton, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
HERMES (NCT04595019) is a novel phase II randomised trial of ultra-hypofractionated stereotactic body radiotherapy (SBRT) in men with localised prostate cancer (1). Treatment is delivered on The Elekta Unity MR-Linac (Elekta AB, Stockholm) a hybrid of a 1.5 Tesla Philips MR and 7MV FFF linear accelerator that enables online adaptive and precise radiotherapy delivery (2).
HERMES randomises between 2 fraction and 5 fraction SBRT2. It opened in September 2021 at The Royal Marsden, with a recruitment target of 46 participants. Figure 1 shows the HERMES trial schematic. Trial outcomes are collected within the MOMENTUM database (3).
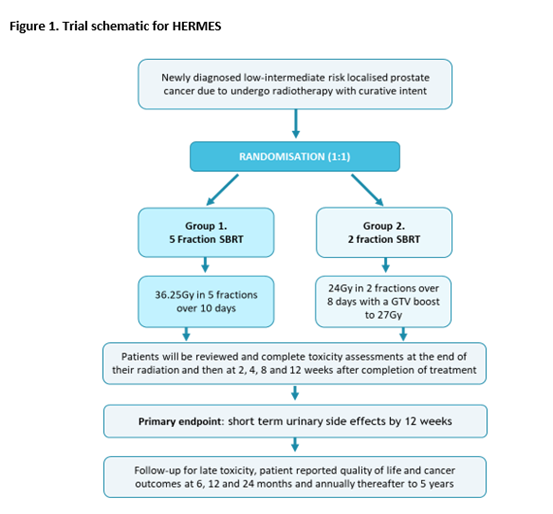
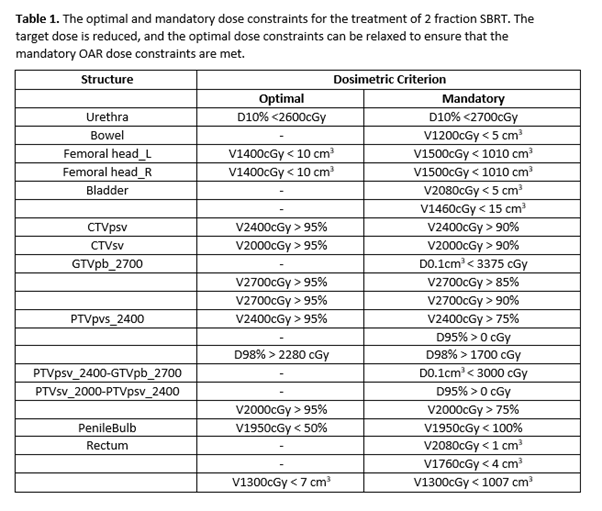
In the experimental arm men are prescribed 24Gy in 2 fractions with a GTV boost of 27Gy. The dose constraints are shown in Table 1.
To promote patient comfort and dose precision each fraction in the 2-fraction arm is delivered in two sub-fractions, delivered sequentially on the same day,
On the day of treatment, the plan is matched to the patient’s position on the Session MRI. The plan is recontoured online using the adapt-to-shape workflow to account for any anatomical changes. A verification MRI is then acquired, and any further deviations in the position prostate accounted for with adapt-to-position prior to treatment delivery.
After sub-fraction 1 the patient gets off the couch to pass urine and waits 15 minutes for bladder filling before getting back on the couch for sub-fraction 2.
We aimed to establish if the plans prescribed for the patients receiving 4 sub-fractions were meeting the clinical goals set out in the protocol.
Material and Methods
20 patients have now been treated within HERMES with 10 patients receiving 2 fraction SBRT.
We reviewed the plans prescribed for each sub-fraction. A total of 40 plans were evaluated from the 10 patients treated with 2 fraction SBRT.
The percentage of all sub-fractions that met the optimal and mandatory dose constraints were calculated.
Results
All 40 sub-fractions treatments were delivered without delay and were well tolerated by patients.
The optimal dose constraint was met in 74% of all cases, where applicable. The mandatory dose constraint was met in 97% of cases across all 40 sub-fractions.
The urethral D10% <2600cGy (+100cGy) was exceeded once, the PTVpsv_2400-GTVpb_2700 in 3 sub-fractions and the rectal V2080cGy < 1 cm3 in 6 sub-fractions (all values taken to 2 decimal places).
Conclusion
2 fraction SBRT to the prostate can be planned online whilst meeting the clinical goals set out in the HERMES protocol.
We plan to report an interim toxicity analysis after the first 20 patients are 12 weeks out from completing their radiotherapy.
Acknowledgments
The HERMES trial is funded by the JP Moulton foundation.
References
1. Westley R et al (2020) https://pubmed.ncbi.nlm.nih.gov/35093251/
2. Winkel D et al (2019) https://pubmed.ncbi.nlm.nih.gov/31341976/
3. de Mol van Otterloo SR et al (2020) https://pubmed.ncbi.nlm.nih.gov/33014774/