Safety & feasibility of SIB in extreme 1-week hypofractionated radiotherapy for early breast cancer
Luis Mateu Castell,
Spain
PO-1297
Abstract
Safety & feasibility of SIB in extreme 1-week hypofractionated radiotherapy for early breast cancer
Authors: Carlos Garcia Zanoguera1,2, Jon Gadea Quinteiro1,2, Alba Galatea Curbelo Artiles1, Luis Mateu Castell1, Irene Ortiz Gonzalez1, Jose Pardo Masferrer1,2
1Hospital Universitario Son Espases, Servicio de Oncología Radioterápica, Islas Baleares, Spain; 2IdiSBa, Institut d’Investigació Sanitaria de les Illes Balears, Islas Baleares, Spain
Show Affiliations
Hide Affiliations
Purpose or Objective
The phase 3 FAST-Forward trial reported that 26Gy in 5 fractions (fx) over 1 week for early-breast cancer patients after breast conserving surgery was non-inferior to the standard 40Gy in 15fx over 3 weeks for local tumor control and normal tissue effects. In this study, a sequential tumor bed boost was allowed (10 or 16Gy in 2Gy/fx) if required. The aim of our study is to evaluate the safety and feasibility of a simultaneous integrated boost (SIB) up to 29/30Gy over 5fx in order to avoid treatment lengthening when a boost is indicated.
Material and Methods
From March 2020 to December 2021, 126 patients eligible for 5fx-RT schedule were referred to our institution and selected for the analysis. A SIB to the tumor bed up to 29Gy in 5fx of 5.8Gy was indicated when any of the following criteria appeared: Age < 60 years, high-grade tumors and lymphovascular or perineural invasion. When positive margins were observed in the histological exam, a SIB up to 30Gy in 5fx of 6Gy was prescribed. Patient´s toxicity was assessed at 4 different points: During RT and at 1, 6 and 12 months follow-up. Toxicity was evaluated using CTCAE 5.0 criteria.
Results
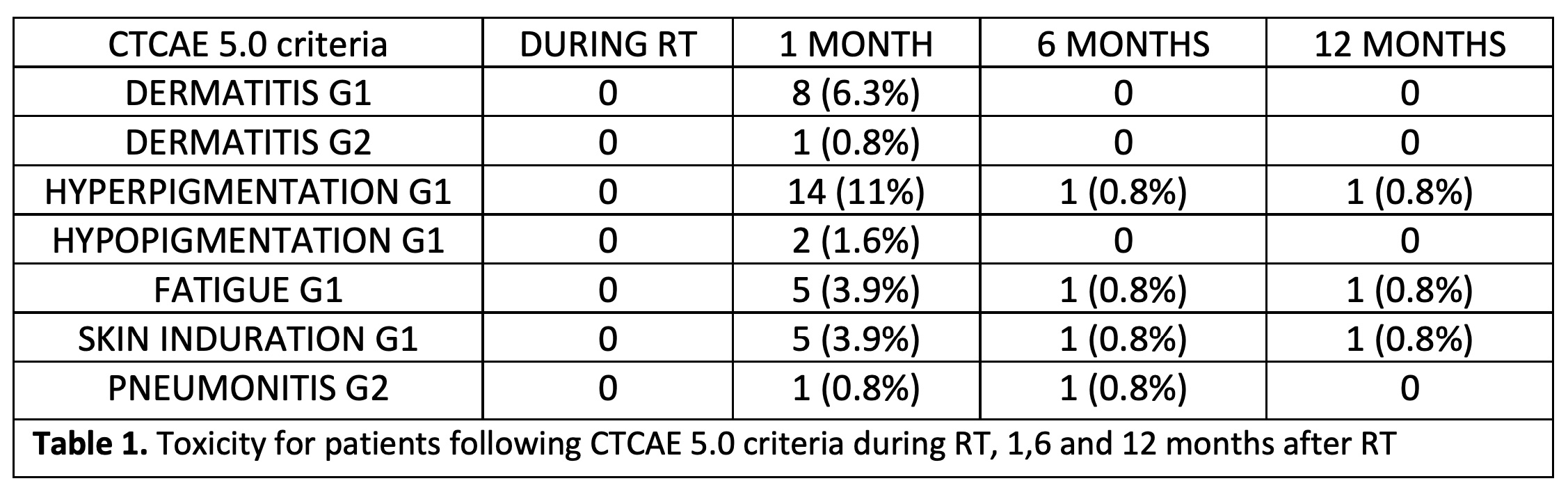
Mean follow-up was 6.25 months and mean age was 56 years (32-83). During RT course, no toxicities were observed. G1-2 dermatitis was observed in 9 patients at first month assessment, but all of them had disappeared at 6 months after RT. According to skin-color changes, 11% of the patients presented skin hyperpigmentation at first month assessment but in most cases was not observed in subsequent evaluations. Only 4% of the patients presented G1 fatigue and skin induration in the first medical visit after RT. No severe toxicities were found in any patient at any time during follow-up. Complete details are shown in table 1. In addition, one patient developed mastitis during the first month after RT but was easily solved with oral antibiotics.
Conclusion
According to our data, administration of SIB up to 29/30Gy in 5fx is safe and feasible for early-breast cancer patients requiring adjuvant RT after breast conserving surgery. SIB could replace sequential boost in these patients shortening the RT treatment and improving patients’ compliance and comfort. Further follow-up is needed in order to assess chronic toxicity.