External validation of the MSKCC nomogram for Ductal Carcinoma in Situ and risk factors analysis
PO-1281
Abstract
External validation of the MSKCC nomogram for Ductal Carcinoma in Situ and risk factors analysis
Authors: Gabriela Oses1, Eduard Mensión2, Claudia Pomarola2, Helena Castillo2, Francesc León1, Inés Torras2, Isaac Cebrecos2, Xavier Caparrós2, Sergi Ganau3, Belén Ubeda3, Xavier Bargalló3, Blanca González-Farre4, Esther Sanfeliu4, Sergi Vidal5, Meritxell Mollà1
1Hospital Clínic of Barcelona, Radiation Oncology, Barcelona, Spain; 2Hospital Clínic of Barcelona, Obstetrics and Gynecology, Barcelona, Spain; 3Hospital Clínic of Barcelona, Radiology, Barcelona, Spain; 4Hospital Clínic of Barcelona, Pathological Anatomy, Barcelona, Spain; 5Hospital Clínic of Barcelona, Nuclear Medicine, Barcelona, Spain
Show Affiliations
Hide Affiliations
Purpose or Objective
Adjuvant radiotherapy and hormonotherapy after breast-conserving surgery (BCS) for ductal carcinoma in situ (DCIS) to prevent local relapse are considered standard of care. To predict the risk of ipsilateral breast tumor relapse (IBTR) after BCS, the Memorial Sloan-Kettering Cancer Center (MSKCC) developed a nomogram. The aim of this study was to evaluate risk factors of local recurrence (LR) in a Spanish cohort and to assess external validation of the MSKCC nomogram.
Material and Methods
A retrospective analysis was carried out between 1999 and 2019, 296 patients were treated for DCIS at the Hospital Clínic of Barcelona. Eighty-nine (30%) patients treated with mastectomy were excluded from the analysis. The following risk factors were analyzed: age, family history, clinical presentation, nuclear grade, pathological tumor , multifocality, comedonecrosis, estrogen and progesterone receptors, final surgical resection margin, number of reinterventions, adjuvant treatments with radiotherapy and hormone therapy. Clinicopathologic and treatment parameters of all patients were used to create a multivariable model and the model of MSKCC was tested. The predictive value of the model was evaluated using Software for Statistics and Data Science release 15.1 (STATA). Statistically significant differences among variables were evaluated as independent risk factors using multivariate analysis with logistic regression.
Results
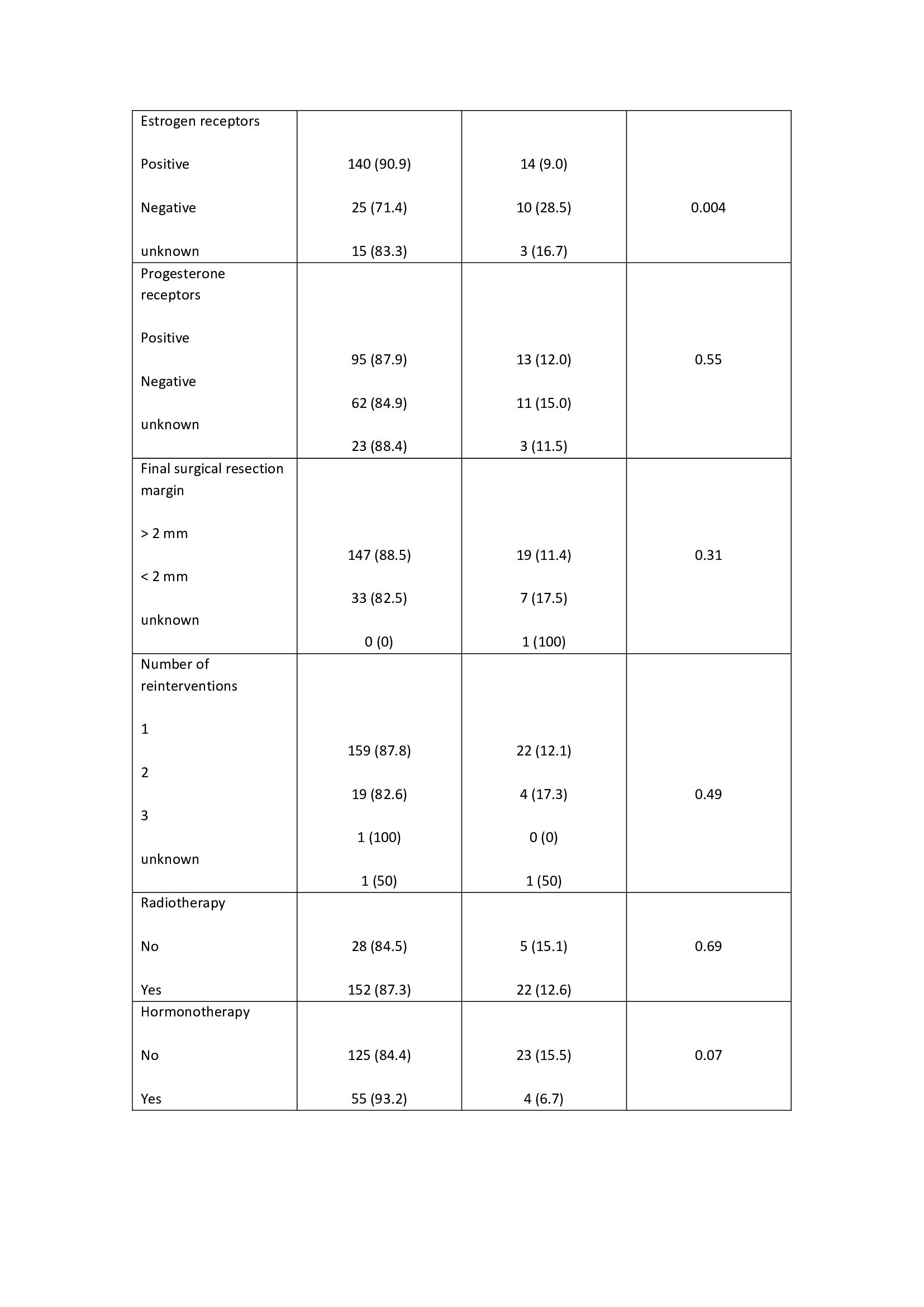
The median follows up time was 10.3 years (1-17), with a mean age of 58 years (42-75). The external validation of the MSKCC nomogram was performed with a total of 207 patients not reaching statistical significance in the studied population to predict LR (p= 0.40). In 7 patients (3.4%) follow-up was lost. The risk factors analyzed are described in table 1. Non-expression of estrogen receptors was significantly associated with an increased risk of LR (p= 0.004). The omission of adjuvant endocrine therapy presented a slight tendency (OR 0.39), but without significance, to an increased risk of LR. The overall local relapse rate was 9.6% (20 patients) during the study period. At the end of the study, 94.6% (196 patients) were alive and 5.4% (11 patients) were dead.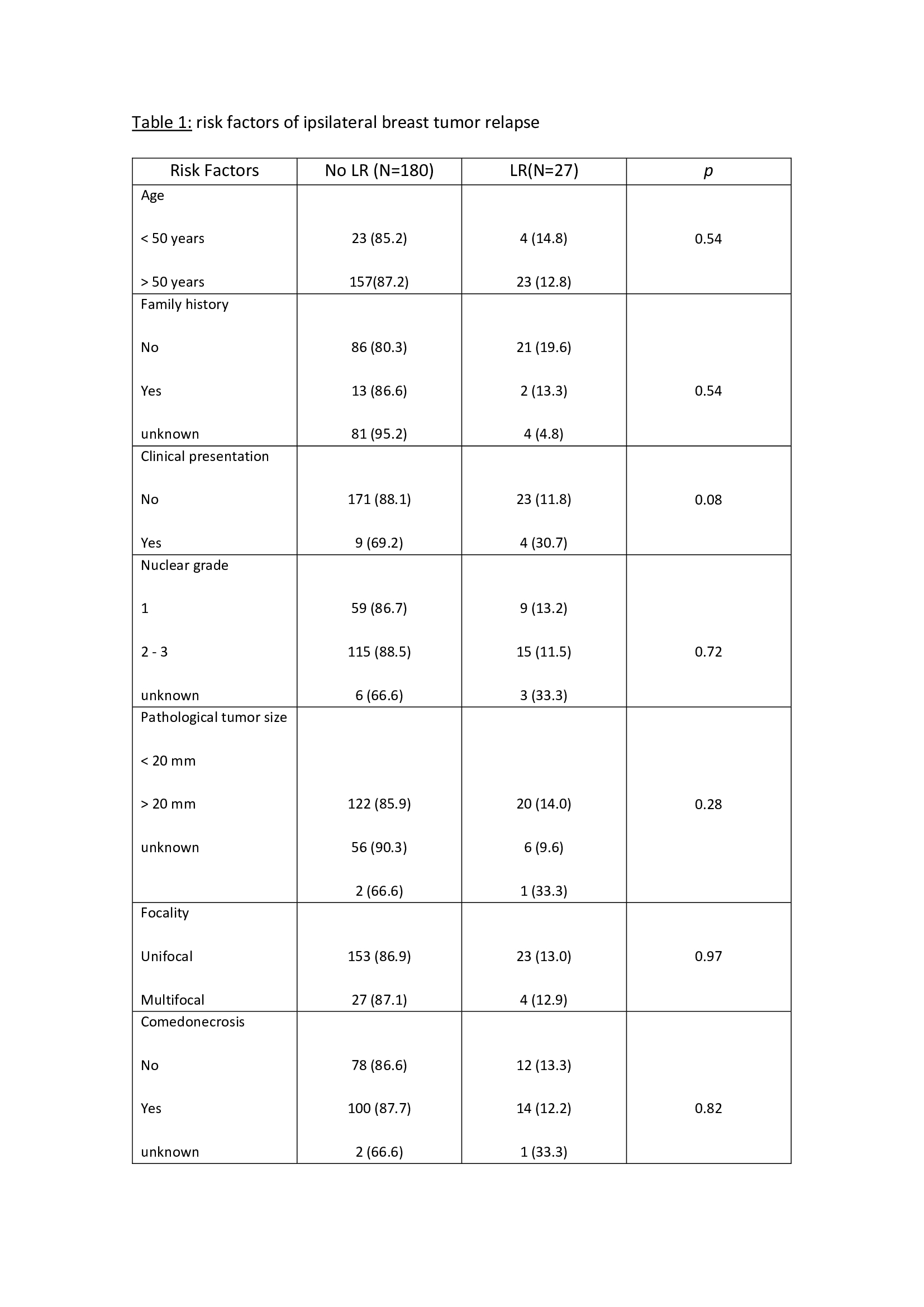
Conclusion
In our series, MSKCC nomogram does not reach statistical significance. Regarding other potential predictive risk factors of LR, non-expression of estrogen receptors was significantly associated with an increased risk of LR. The LR rate was 9.6%, in accordance with the published series.