Replacing patient-specific pretreatment measurements for lung SBRT by 3D secondary dose calculation
Markus Wendling,
The Netherlands
PO-1649
Abstract
Replacing patient-specific pretreatment measurements for lung SBRT by 3D secondary dose calculation
Authors: Markus Wendling1, Thierry Felkers1, Jeroen Findhammer1, Ruud van Leeuwen1
1Radboud university medical center, Radiation Oncology, Nijmegen, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Pretreatment
dose verification of stereotactic body radiotherapy (SBRT) is generally
considered necessary because of its high accuracy requirements. These
measurements come at the cost of extra work that must be balanced with their capability of detecting critical plans. Also secondary dose calculations (SDCs)
can be used for finding (large) errors in the dose calculation of a plan,
whereas the extra workload for using these SDC systems is limited.
In our
clinic the question arose whether the number of pretreatment measurements for lung SBRT can
be reduced. Whereas pretreatment measurements for lung SBRT are evaluated at g criteria of 3%/3mm, the 3D SDC is generally
done at 5%/3 mm. The aim of the study was to develop a method to recalculate a
large number of lung SBRT plans with the 3D SDC at the stricter 3%/3mm criteria
and to judge if pretreatment measurements can be minimized.
Material and Methods
Plans of patients
treated with lung SBRT from January 2020 to August 2021 were taken from the clinical
database. No other selection criteria were applied. The plans were made with
Pinnacle TPS (Philips, USA) using VMAT arcs of 10 MV photons. All plans were
verified pretreatment on a Delta4 phantom (ScandiDos, Sweden) and fulfilled our
clinical g criteria of
3%/3mm with a passing rate ≥95% at a 40% dose threshold. Additionally, all
plans passed our clinical 3D SDC (Mobius3D, Varian Medical Systems, USA) using
our clinical g criteria of
5%/3mm with a passing rate ≥95% at a 40% dose threshold.
The selected
plans were recalculated with the 3D SDC using criteria of 3%/3mm. In order not
to interfere with the standard workflow, a dicom tag was modified during
transfer from our radiotherapy PACS to the 3D SDC and that triggered the use of
the tighter settings. Results were automatically stored.
Results
136 unique lung
SBRT plans, including patients with multiple lesions, were automatically recalculated
at 3%/3 mm with the 3D SDC. 129 of the plans (95%) had a g passing rate ≥95%. Although the
treatments were heterogenous in prescription and fractionation, the histogram
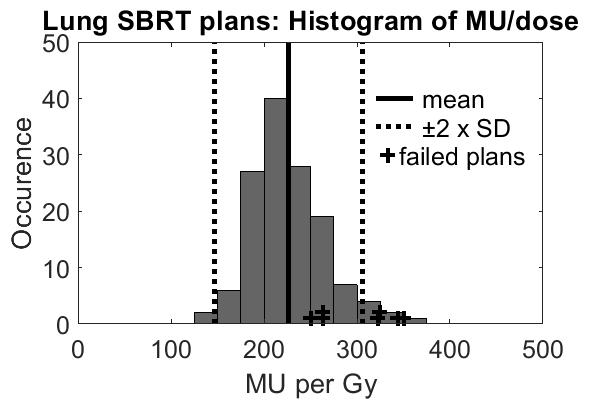
of (monitor units)/(prescribed dose) had a relatively small distribution (see fig.).
The plans that failed the criteria are indicated in the figure; 4 of the 7
failed plans (57%) were “in the tails” of the distribution.
Conclusion
A large
number of lung SBRT plans was re-evaluated using our 3D SDC at the same
criteria as for pretreatment measurements. The majority of plans (95%) passed
these criteria. It is often recommended to do extra QA for plans that are more complex than the “standard” plans. However, by using (monitor units)/(prescribed
dose) as plan complexity measure, 3 plans would have escaped extra QA.
By using
tighter 3D SDC criteria potentially critical plans can be identified. We recommend a risk-based selection for pretreatment QA by measuring
those plans that do not meet the pretreatment criteria in the 3D SDC and to
combine this with sampling the rest of the plans. This will result in a vast
reduction of work load.