Cell kill based treatment planning for polymetastatic patients with high tumor burden
Nathan Torelli,
Switzerland
PO-1648
Abstract
Cell kill based treatment planning for polymetastatic patients with high tumor burden
Authors: Nathan Torelli1, Yuting Wang1, Simon Burgermeister1, Hubert S. Gabryś1, Indira Madani1, Matthias Guckenberger1, Jan Unkelbach1
1University Hospital Zurich and University of Zurich, Department of Radiation Oncology, Zurich, Switzerland
Show Affiliations
Hide Affiliations
Purpose or Objective
In
patients with oligometastatic diseases, the early integration of local metastases
directed treatment into a multimodality treatment strategy has shown improved
clinical outcomes and is nowadays considered a standard treatment option.
Recently, it has been proposed to transfer the principles of oligometastatic
disease into treatment of polymetastatic cancer patients. Although local
ablation of all visible metastases may not be feasible in this situation due to
dosimetric constraints, it is hypothesized that early and maximum local
consolidative radiotherapy (MLCR) of all metastatic lesions which do not achieve
complete response to systemic therapy, may delay drug resistance development and
disease progression. Traditional radiotherapy planning approaches, in which
each metastasis is treated to the same dose of radiotherapy every day, are
however not suitable with respect to safety and efficacy for targeting multiple
metastases across the body. In the context of MLCR for polymetastatic cancer
patients, in fact, it is a priori unclear what dose can or should be prescribed
to each metastasis. To address this problem, we implemented a radiotherapy
planning approach which simultaneously minimizes the total number of surviving
tumor cells and preserves organ function.
Material and Methods
An
exponential cell survival model was assumed according to which the total number
of surviving tumor cell is
Ʃm ƩiϵPTVm c
exp(-α
di)
where di is the dose in voxel i,
α is
the radiosensitivity parameter, and we sum over all voxels i that belong
to any of the metastases m. This was used as an objective function for
IMRT planning, while constraining the mean lung dose to 9 Gy and V20Gy
to 10% in a 5-fraction SBRT regimen. Such a radiotherapy planning approach was investigated
for three metastatic melanoma patients with a varying number of lung metastases.
Results
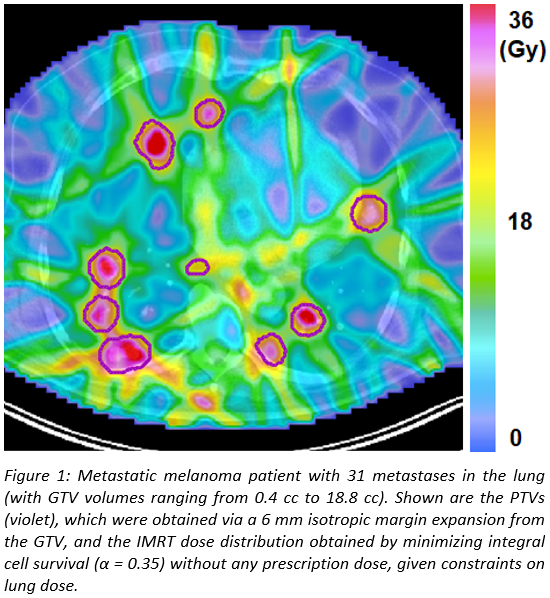
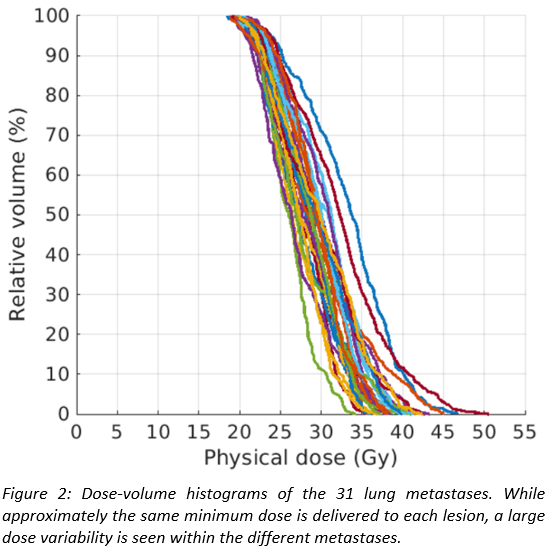
For
a patient with 31 lung metastases, Figures 1 and 2 show the dose distribution
and the DVHs of the individual lesions, respectively. The method delivers
similar minimum doses of approximately 18 Gy to all metastases, where the value
of the minimum dose is determined by lung constraints. However, inhomogeneous
doses are delivered within each lesion, with the mean dose varying between the individual
metastases by up to 10 Gy. The method exploits the fact that for the same
increase in lung dose, lesions located in favourable positions can be irradiated
to higher radiation doses than others, and thereby increases overall cell kill
for a given lung dose constraint compared to a fixed prescribed dose to all
metastases.
Conclusion
The
proposed biological cell-killing based radiotherapy planning approach allows
for personalized treatments of patients with wide-spread metastatic disease,
thereby overcoming the limitations of traditional planning approaches of
delivering the same homogeneous radiotherapy dose to each lesion, regardless of
the individual metastasis location, size and relationship to critical organs at
risk.