Reduction of second cancer risks with proton therapy vs photon VMAT in seminoma patients
Wilma Heemsbergen,
The Netherlands
PO-1518
Abstract
Reduction of second cancer risks with proton therapy vs photon VMAT in seminoma patients
Authors: Wilma Heemsbergen1, Maarten Dirkx1, Denise De Regt2, Martine Franckena1, Yvonne Klaver3, Steven Habraken3, Remi Nout1
1Erasmus MC Cancer Institute, Radiotherapy, Rotterdam, The Netherlands; 2InHolland , University of Applied Science, Haarlem, The Netherlands; 3Holland Proton Therapy Center, Radiotherapy, Delft, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Elective para-aortic (PAO) radiotherapy (RT) has
been the standard adjuvant therapy after local surgery in stage I seminoma for
many years, using photons and an APPA field setup. In the past decade this
policy is however shifting towards watchful waiting, since several cohort
studies demonstrated that this young patient category with excellent overall
survival is at considerable risk for late effects including second primary cancer
(SPC) risks related to the radiation exposure of organs at risk (OAR), in
particular the stomach, pancreas, and kidneys. Current developments in RT offer
more advanced options with VMAT and proton therapy, with the potential to
reduce OAR dose levels and therefore SPC risks. In the current study we
compared dose to the OARs between VMAT, proton therapy and an APPA reference
group. Mean dose was considered as a suitable proxy for SPC risks, since a
linear dose-response is assumed in the observed OAR dose range of ≈0.1-15 Gy, causing cell damage but not cell kill, according to the theoretical models (linear, linear plateau, linear exponential).
Material and Methods
In six recent
clinical treatment plans we added delineations of pancreas and stomach and
re-optimized the VMAT plan (13x2 Gy, single-ARC, 10 MV, clinical constraints)
applying a uniform 7 mm PTV-CTV margin and created an additional proton plan.
All received RT to the PAO lymph nodes and two had additional RT to
para-iliacal (PAI) lymph nodes. Proton therapy plans were optimized using
in-house developed software for the prioritized multi-criteria optimization of RT
treatment plans. A standard setup of 2 posterior beams at gantry angles of 165
and 195 degrees were used. Scenario-based robust optimization with 7mm setup
error and 3% relative stopping power prediction error (range) robustness was
used.
Results
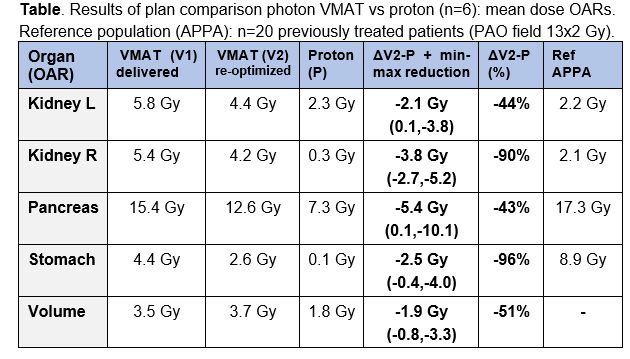
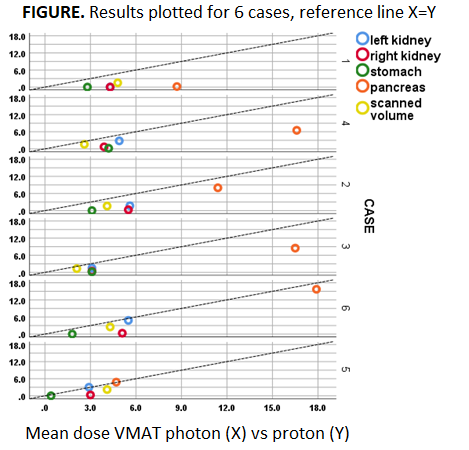
Mean dose to OARs
and the total scanned volume (proton vs VMAT) showed relevant OAR dose reductions
for the majority of patients and for all OARs, with largest absolute reductions
for pancreas and right kidney (4-6 Gy), and largest relative reductions for
stomach and right kidney (90%-96%); see Figure & Table. The total sum of
mean dose reductions (kidney L + kidney R + stomach + pancreas) was in the
range of 15-20 Gy in 4 patients (all PAO), 10 Gy in 1 patient and 3 Gy in 1
patient (both PAO+PAI: case nr 5 & 6 in Figure). Compared to the historical
APPA group, we observed that with VMAT dose levels to the stomach decreased and
dose to the kidneys increased. With proton therapy, low dose levels to both
kidney and stomach can be achieved.
Conclusion
We demonstrated
that with proton therapy clinically meaningful OAR dose reductions can be
achieved with respect to SPC risks in a proportion of the seminoma patient
group, compared to modern RT with VMAT. Risk reductions of other late effects
(e.g. diabetes) can be expected as well. For young seminoma patients with
unfavorable dose distributions to OARs with a VMAT plan, proton therapy would
therefore be a superior treatment option.