Risk stratification based on whole body tumor burden can refine the role of combining RT and ICIs
Jina Kim,
Korea Republic of
PO-1435
Abstract
Risk stratification based on whole body tumor burden can refine the role of combining RT and ICIs
Authors: Jina Kim1, Jin Sung Kim1, Heejoo Ko2, Tae Hyung Kim3, Seo Hee Choi4, Wonmo Sung5, Sang Joon Shin6, Jee Suk Chang1
1Yonsei University College of Medicine, Radiation Oncology, Seoul, Korea Republic of; 2The Catholic University of Korea, Collegeof Medicine, Seoul, Korea Republic of; 3Eulji University School of Medicine, Radiation Oncology, Seoul, Korea Republic of; 4Yongin Severance Hospital, Yonsei University College of Medicine, Radiation Oncology, Yongin, Korea Republic of; 5The Catholic University College of Medicine, Biomedical Engineering, Seoul, Korea Republic of; 6Yonsei University College of Medicine, Division of Medical Oncology, Department of Internal Medicine, Seoul, Korea Republic of
Show Affiliations
Hide Affiliations
Purpose or Objective
Two recent trials showed conflicting findings of adding RT to immune checkpoint inhibitors (ICI) in unselected patients with metastatic disease. Although the current definition of oligometastatic disease is based on the number of metastases and involved organs, little is known about which quantitative methods (lesion number and sum of 2D or 3D measurements) are suitable for better risk stratification in RT and ICI combination trials. Here, we investigated the clinical utility of different quantitative characteristics of metastatic disease in advanced melanoma patients who received pembrolizumab.
Material and Methods
86 patients with metastatic or unresectable malignant melanoma were treated with pembrolizumab between 2015 and 2020. We 3-dimensionally contoured all metastases on available imaging at the time of pembrolizumab initiation. Then, total number of metastases, sum of 10 target lesions per RECIST criteria, 3D volume of total gross tumors and involved organs (liver, lung, brain, and bone) were collected for analysis. The study endpoints included overall survival (OS), progression-free survival (PFS) and PFS-2 (progression after the next line of therapy).
Results
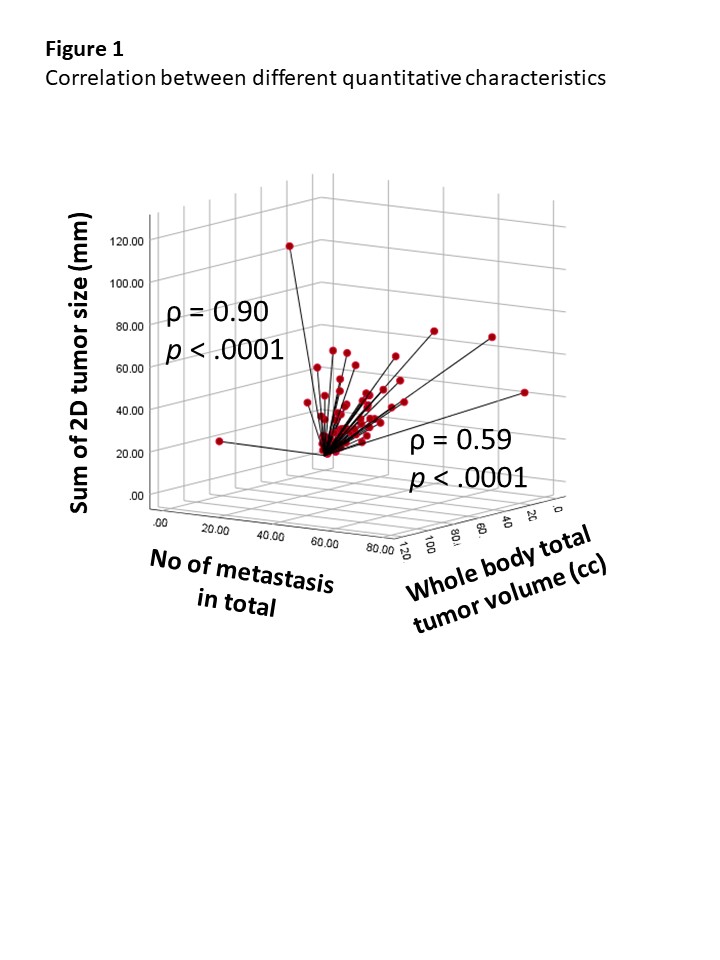
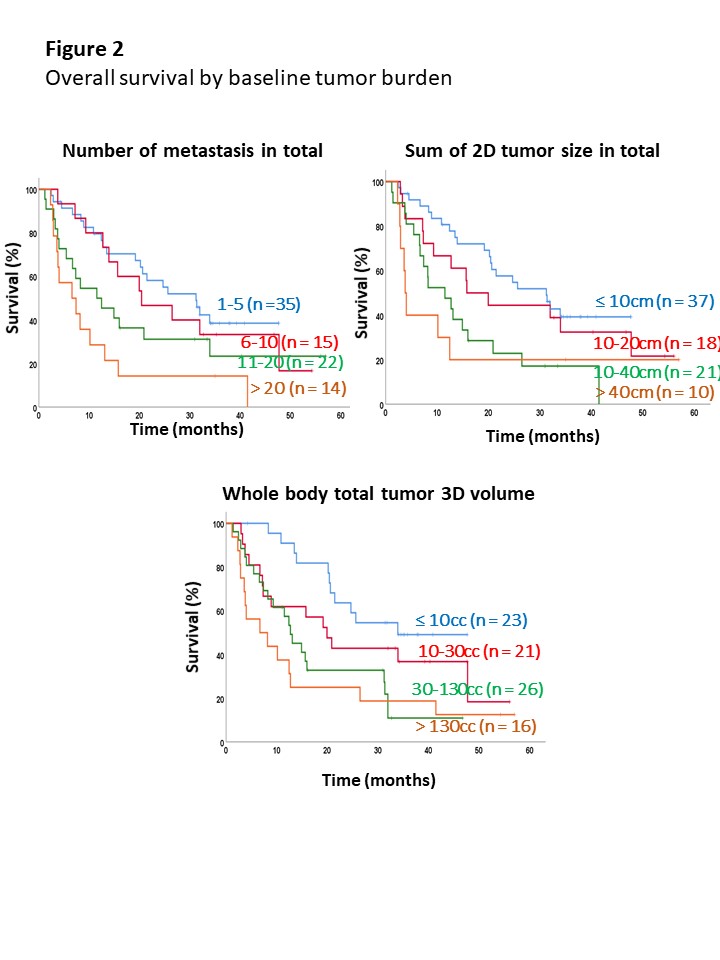
In total, 1049 metastases were contoured and the average number, 2D-tumor size, and 3D-tumor volume of total metastases before treatment initiation were 12±14/patient, 12.8±20.19 cm/patient, and 94±183 cc/patient, respectively. High correlation between the number of metastatic lesions and baseline 2D-tumor size was observed (ρ = 0.90), but moderate correlation was seen between the number of metastatic lesions and 3D-tumor volume (ρ = 0.59). At a median follow-up of 15.9 (range, 1.2-57.0) months, the number of metastatic lesions, 2D-tumor size, and 3D-tumor volume showed significant correlation with OS, PFS and PFS-2 in a continuous manner (e.g., 1-5, 6-10, 11-20 and >20; ≤ 10 cm, 10-20 cm, 20-40 cm and >40 cm; and ≤ 10 cc, 10-30 cc, 30-130 cc and > 130cc, respectively). In multivariate analysis, tumor involvement in liver (hazard ratio [HR] 1.79) and brain (HR 5.52) were independently associated with poor OS along with quantitative characteristics.
Conclusion
Through this hypothesis-generating study, we demonstrated the prognostic value of baseline tumor burden and metastasis locations in metastatic melanoma patients receiving pembrolizumab. Our results are helpful for better risk stratifying and designing clinical study which refines the role of RT in combination with immunotherapy.