SBRT monotherapy for low and intermediate risk Prostate Cancer. First Chilean experience.
María Fernanda Sánchez,
Chile
PO-1392
Abstract
SBRT monotherapy for low and intermediate risk Prostate Cancer. First Chilean experience.
Authors: María Fernanda Sánchez1, Felipe Maza2, Federico Bakal1, Piero Bettoli1
1Fundación Arturo López Pérez, Department of Radiation Oncology, Santiago, Chile; 2Fundación Arturo López Pérez, Unidad de Evaluación de Tecnologías Sanitarias, Santiago, Chile
Show Affiliations
Hide Affiliations
Purpose or Objective
Recent studies have demonstrated both safety and efficacy of SBRT as monotherapy in the treatment of low and intermediate risk prostate cancer. The aim of this series is to present the first Chilean experience with SBRT in the treatment of localized prostate cancer.
Material and Methods
Low and Intermediate-risk prostate cancer treated within our SBRT protocol were prospectively assessed. For intermediate-risk patients a ≤10% lymph node risk according to MSKCC nodal risk nomogram was mandatory for inclusion. Fiducial-based image-guided SBRT was delivered with the CyberKnife M6 FIM version 10.6 (Accuray Inc, Sunnyvale, CA). Treatment planning was performed using a CT scan with MRI fusion. All patients were treated with a dose of 36.25 Gy given in 5 consecutive fractions. Androgen deprivation hormonal therapy (ADT) was allowed only in the intermediate risk group as per physician’s discretion. Biochemical progression-free survival (bPFS) was calculated using the Phoenix definition. Patient self-reported QOL was prospectively measured using the Expanded Prostate Cancer Index Composite (EPIC-26) instrument at baseline and at regular intervals. One half standard deviation below the baseline was considered a minimally important differences (MID) for an individual patient's change in each domain. Acute and late genitourinary (GU) and gastrointestinal (GI) toxicities were graded using CTCAE version 5.0.
Results
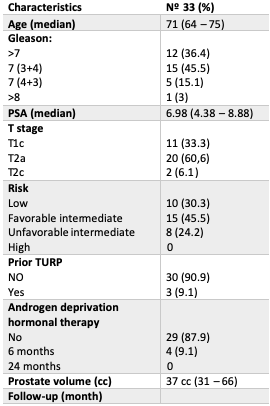
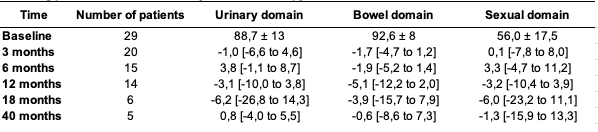
After a median follow-up of 24 months (IQR 12-40) thirty-three patients were eligible for analysis. Median age of our cohort was 71 years (IQR 64-75). Median prostate volume was 37 cc (IQR 31-66) and median baseline IPSS score 6 (IQR 3-10). Three patients had previous history of TURP. 30.3% (n=10), 45.5% (n=15) and 24.2% (n=8) of the patients were classified as low-risk (LR), favorable-intermediate-risk (FIR) and unfavorable-intermediate-risk (UIR) group according to the NCCN risk classification respectively. 12% (n=4) of the patients in the UIR group received 6 months of ADT. Actuarial 3-year bPFS was 97% for the entire cohort. For urinary and bowel QoL domain, minimal fluctuations above MID threshold were observed during follow-up except for one slightly deviation below MID at 12 months in bowel QoL domain (MID -5.1). For sexual QoL domain, the observed decline remained stable within MID threshold. Treatment was well tolerated with no acute Grade 3 ≥ GI or GU toxicities. No late Grade 3 GI or GU toxicity were observed. One patient developed a rectourethral fistula (GI Grade 4 toxicity) in the context of a biopsy of a rectal ulcer performed 12 months after SBRT.
Conclusion
SBRT monotherapy for low and intermediate risk prostate cancer patients is well-tolerated, has little impact on health related QOL and reveals excellent early bPFS which is in line with current body of literature. Post-SBRT interventions such as rectal biopsy may increase the risk of fistulas and should be avoided.