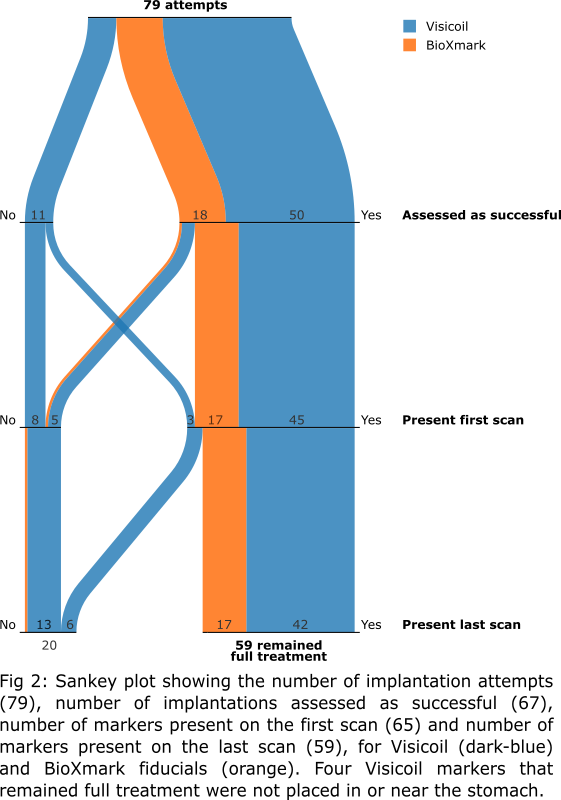Feasibility of endoscopic fiducial marker implantation in the stomach for use in image-guided RT
Margot Bleeker,
The Netherlands
PO-1308
Abstract
Feasibility of endoscopic fiducial marker implantation in the stomach for use in image-guided RT
Authors: Margot Bleeker1, Roos Pouw2, Arjan Bel1, Jan-Jakob Sonke3, Maarten Hulshof1, Astrid van der Horst1
1Amsterdam UMC, University of Amsterdam, Department of Radiation Oncology, Amsterdam, The Netherlands; 2Amsterdam UMC, Vrije Universiteit Amsterdam, Department of Gastroenterology and Hepatology, Amsterdam, The Netherlands; 3The Netherlands Cancer Institute, Department of Radiation Oncology, Amsterdam, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Fiducial markers can assist in target
localization during cone beam CT (CBCT) guided radiotherapy. The purpose of
this study is to assess technical feasibility of fiducial marker implantation
for use in pre-operative gastric cancer radiotherapy.
Material and Methods
Fiducial markers were placed endoscopically in the stomach of
gastric cancer patients (MaagART-01 trial, No. NL7036) prior to radiation treatment
(45Gy in 25 fractions; daily CBCT imaging). Each procedure was performed under mild
or deep sedation by one of four gastroenterologists. All of the currently 12
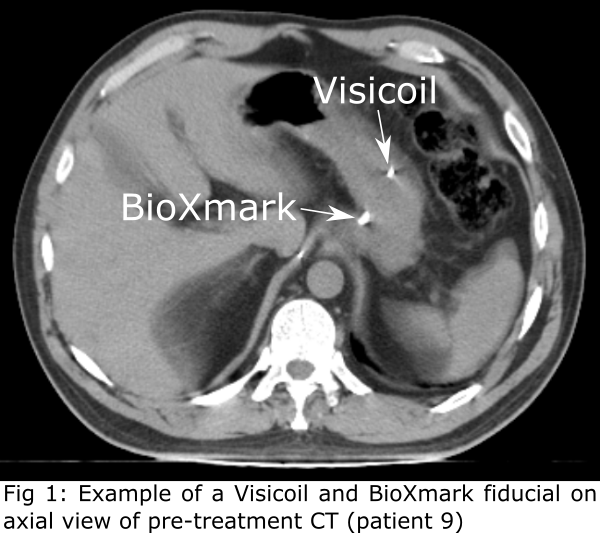
included patients received gold markers (Visicoil, Core Oncology, CA, USA; Ø
0.35mm x 10mm length), which were individually backloaded into a 22-gauge endoscopic
needle prior to each placement. The last 5 patients also received liquid
markers (BioXmark, Nanovi, Kongens Lyngby, Denmark; injection volume 0.08−0.20 mL).
The liquid marker was loaded once per patient in a 25-gauge injection needle,
allowing placement of multiple subsequent markers without retraction of the
needle.
We evaluated duration of
implantation (from first loaded needle entering the endoscope to final fiducial
placed) and occurrence of complications (e.g. bleeding, perforation, fever). For
each marker, we determined whether (a) its implantation was assessed as successful
at time of implantation (i.e., marker secured in tissue), (b) it was present on
the first scan post-implantation (pre-treatment CT or first CBCT (on
reconstructed CBCT or projection images)) and (c) it was present on the last
fraction’s CBCT. Finally, we assessed adequacy of location, i.e. in or near the
stomach wall.
Results
In total, we attempted to implant
79 markers (5−8 per patient; 61 Visicoil and 18 BioXmark; Fig 1).
Duration of implantation was 16−38 min (mean=24 min), implantation
time per marker was 2.5−5.4 min (mean=3.7 min). There were no procedure-related complications.
At time of implantation, 68 markers were assessed to be implanted
successfully (Fig
2).
When markers were assessed as not successful at time of implantation (all
Visicoil), this was mostly due to either technical difficulties (e.g. unable to
push marker out of needle), or incomplete implantation caused by peristaltic
motion.
On the first scan
post-implantation, 65 markers were present. Of those, 59 were still present on
the last fraction’s CBCT, 4 disappeared prior to radiation treatment (within 1−10
days post-implantation), and 2 disappeared during treatment (15 and 22 days
post-implantation; fractions 5 and 8). Four markers (all Visicoil) were not
placed correctly: one in diaphragm, one in spleen, and two in fatty tissue
>1 cm from the stomach. For the markers that remained full treatment, no
migration was observed.
Conclusion
Fiducial marker placement in the
stomach was technically feasible and successful, for both Visicoil (38 of 61
attempts, 62%) and BioXmark (17 of 18, 94%). Visibility and benefit of fiducial
markers in image-guided radiotherapy (using 4DCT and 4DCBCT) will be evaluated
in an ongoing study.