Prospective phase 2 study of induction FOLFIRINOX followed by chemoradiation in LA-Pancreatic Cancer
Gian Marco Petrianni ,
Italy
PO-1303
Abstract
Prospective phase 2 study of induction FOLFIRINOX followed by chemoradiation in LA-Pancreatic Cancer
Authors: Gian Marco Petrianni1, Michele Fiore1, Pasquale Trecca1, Gabriele D'Ercole1, Luca Eolo Trodella1, Carlo Greco1, Edy Ippolito1, Damiano Caputo2, Roberto Coppola2, Sara Ramella1
1University Campus Bio-Medico of Rome, Radiotherapy, Rome, Italy; 2University Campus Bio-Medico of Rome, General Surgery, Rome, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
The aim of
this study was to evaluate the safety and efficacy of induction treatments in
patients with borderline resectable or unresectable locally
advanced pancreatic cancer and the efficacy of pre-operatory staging with 18FDG
PET-CT and laparoscopy in addition to CT scan.
Material and Methods
From 2015 to 2021
we evaluated 42 patients with
borderline resectable or unresectable pancreatic cancer. A
pre-treatment staging was performed with CT scan, 18FDG PET-CT scan and
laparoscopy. Patients with metastatic disease were excluded. Suitable patients
received induction treatments with FOLFIRINOX. After 4 cycles patients were
restaged with CT scan and 18FDG PET-CT scan. Patients without evidence of
metastatic disease started chemoradiation (CRT) with weekly
gemcitabine. After CRT, before surgery evaluation, patients performed CT
scan and 18-FDG PET-CT scan.
Results
Fifteen patients
(39.5%) were excluded from the protocol because of the evidence of metastatic
disease, and thus a total of twenty-three patients were consequently enrolled.
Four patients (14.8%) had a progression of disease after induction
chemotherapy. Median follow-up was 12.6 months. Nineteen patients (50%)
completed CRT. Six patients (15.8%) had a progression of disease
after CRT. Four patients are currently treating. Eleven patients underwent
surgical radical resection (28.9%) (Fig.1). The median OS and the median PFS in
patients who completed the therapeutic protocol were 15.7 months and 13
months, respectively. One-year OS, one-year PFS, one-year LPFS and one-year
MPFS were 87.1%, 58.6%, 89.2% and 60%, respectively. Patients who underwent
resection had a significant longer median OS compared with non-resected
patients (17 months vs 13.2 months, p<0.05). The median PFS for resected
patients was 14.5 months compared with 8.1 months for non-resected patients
(p=0.07). For the entire cohort of patients the treatment was well tolerated.
Only haematological grade 3-4 toxicities were observed.
Fig.1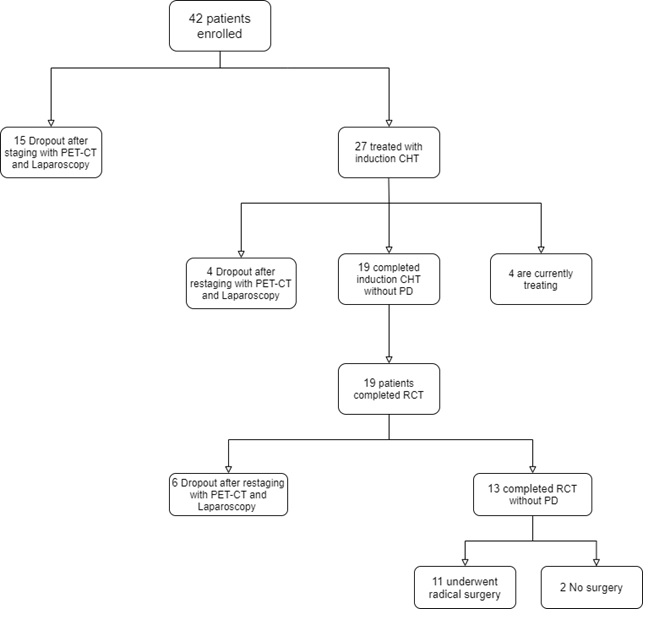
Conclusion
Altough the
follow-up time is limited, these preliminary data of the protocol treatment
show promising results for patients with borderline resectable and unresectable pancreatic
cancer. The best results were observed in patients who
were resectable after the end of the study protocol. The
enrollment is actually ongoing.