n = 89. Median age 74 years (50 - 90), 49.5% of whom were male and 50.5% were female. Figure 1 summarises demographics of patients and tumour characteristics. 98.8% of patients had T1-2 tumours and 89.9% underwent SABR with 55Gy/5# fractionation.
Dosimetric data analysis showed that the mean planning target volume (PTV) (cc) was 26.6 and the median was 21.5. In subgroup analysis, patients treated with 55Gy/5# and 60Gy/8#, the median PTV was 48.1cc and 23.08cc respectively. The PTV was larger in range and larger on average in those treated with 5 fractions (mean PTV 26.6cc) as opposed to those treated with 8 (mean PTV 25.3cc). The median values for R100, R50, and D2cm were 1.1 (0.21 - 1.63), 6 (4.1 - 13.9) and 31.4 Gy (range 21.1 - 45.18). The median mean lung dose and V20 were 3.3 Gy (1.68 Gy - 6.3 Gy) and 4.15 % (1.29 % - 8.7 %) respectively. The median PTV maximum and minimum doses (Gy) were 74.85 (19.19 - 83.8) and 47.8 (29.66 - 71.9) respectively. The PTV V90% and PTV V100% doses were 99.9 (30.27 - 100) and 95.015 (20.8 - 99.66).
Patients had an overall median survival of 52.2 months (Kaplan-Meier survival analysis). Patients surviving to <1 year, 1 year, 2 years, 3 years, 4 years and 5 years and 6 years or more being 94.4%, 79.8%, 68.5%, 60.7%, 24.72% and 11.23% respectively.
9% of patients had local, 5.6% had regional and 14.6% had distant recurrent disease, 67% of patients had no recurrence. Of the variables examined, only the PTV volume and PTV minimum dose had an impact on overall survival on both uni- and multi-variate cox proportional analysis (Figure 2).
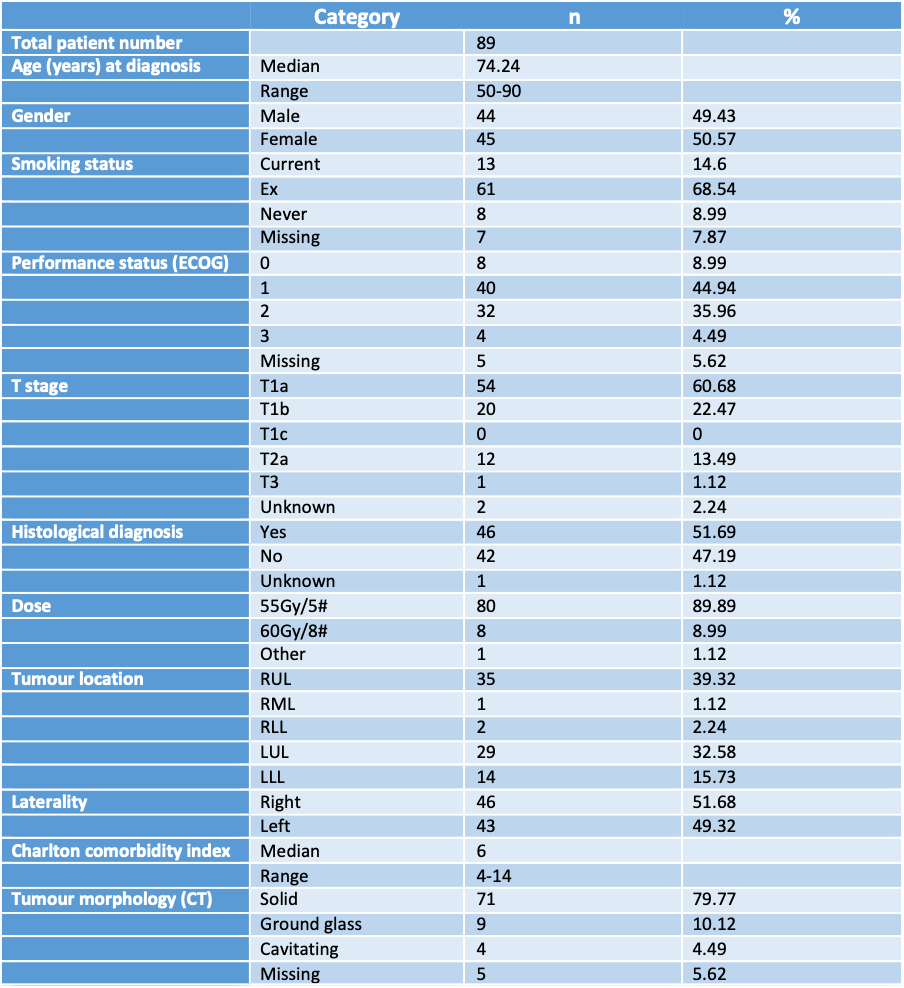
Figure 1. Patient demographics and tumour characteristics
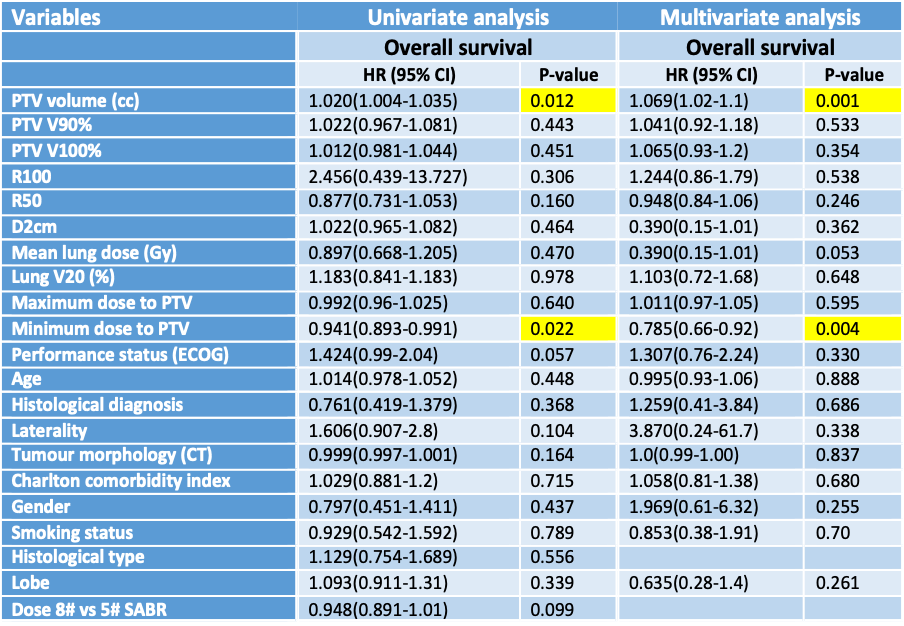
Figure 2. Uni- and multi-variate cox proportional hazard analysis correlating clinical, pathological and dosimetric variables with outcomes