Stereotactic radiotherapy to primary breast cancer in de novo stage IV disease: preliminary results
PO-1186
Abstract
Stereotactic radiotherapy to primary breast cancer in de novo stage IV disease: preliminary results
Authors: Edy Ippolito1, Sonia Silipigni1, Paolo Matteucci1, Sofia Carrafiello1, Michele Fiore1, Carlo Greco1, Alessia Di Donato1, Vincenzo Palumbo1, Claudia Tacconi1, Sara Ramella1
1Campus Bio-Medico University, Radiation Oncology, Rome, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
Approximately
5-10% of women present de novo metastatic breast cancer (MBC). Even if current
literature data do not support loco-regional treatment in all patients, some of
the study results suggest that there is a subset of patients who might benefit
from radical locoregional treatment especially those patients who have
significantly improved survival with the available new effective target agents.
Potential advantages of stereotactic ablative radiotherapy (SABRT) in treating
breast primary tumor in metastatic breast cancer relies on the radio-biological
advantage of a short highly effective treatment schedule, the possibility of
continuing systemic treatment without interruption and of treating symptomatic
lesions. We developed a prospective dose escalation trial to evaluate the maximum tolerated dose of SABRT to
primary breast cancer in this setting
Material and Methods
Patients with histologically confirmed diagnosis of invasive breast
carcinoma (biological immunohistochemical profile: luminal and/or HER2
positive) and distant metastatic disease not progressing after 6 months of
systemic therapy with a tumor CT or 5FDG-PET detectable were deemed eligible. The
starting dose was 40 Gy in 5 fractions (level 1) because this dose proved to be
safe in previous dose-escalation trial on adjuvant stereotactic body
radiotherapy. The maximum dose level was chosen as 45 Gy in 5 fractions. Dose
limiting toxicity was any grade 3 or worse toxicity according to CTCAE v.4. Time-to-event Keyboard (TITE-Keyboard) design (Lin and Yuan,
Biostatistics 2019) was used to find the maximum tolerated dose (MTD). MTD was
the dose of radiotherapy associated with a ≤ 20% rate pre-specified
treatment-related dose –limiting toxicity (DLT).
Results
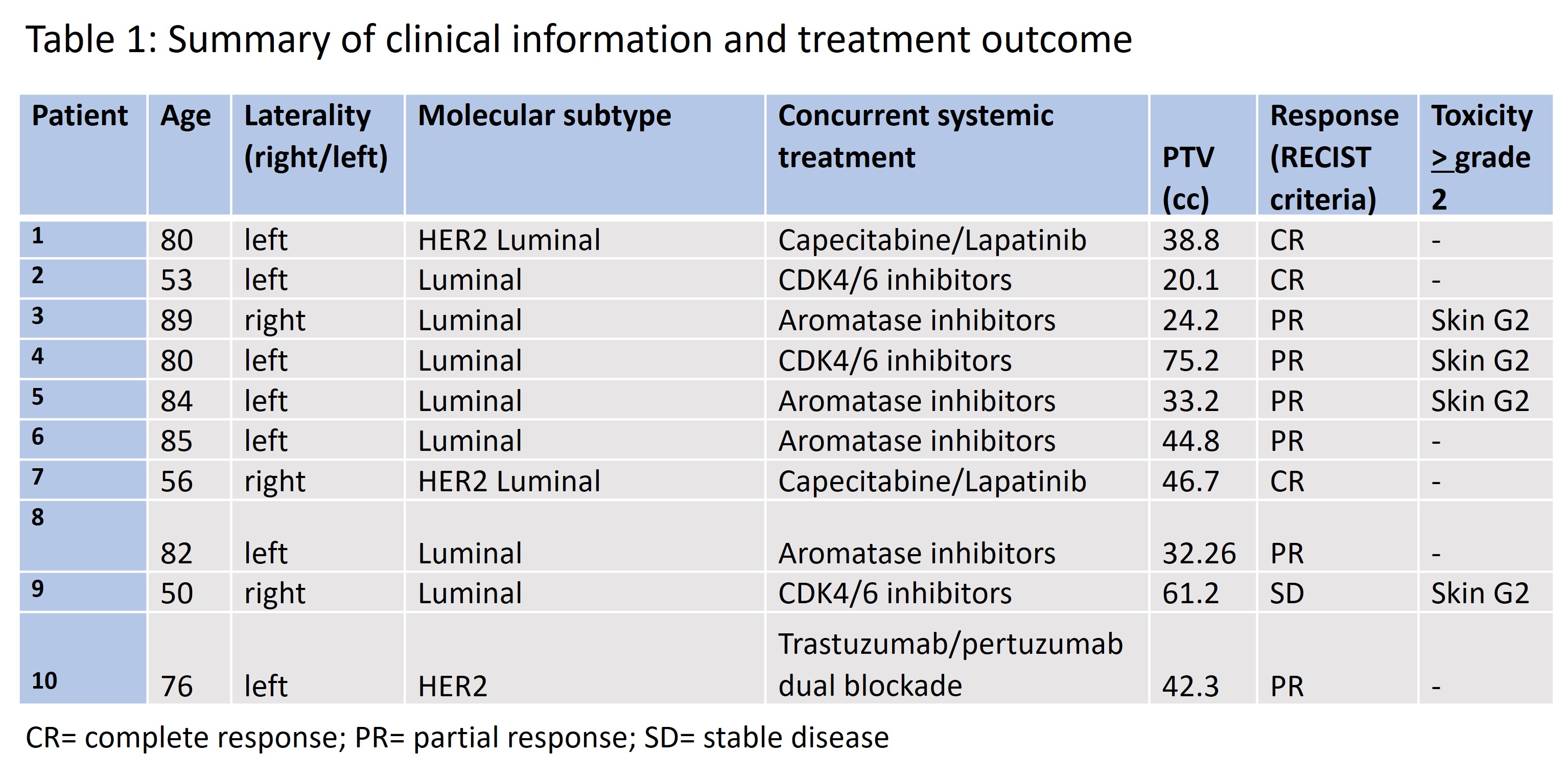
To
date 10 patients have been treated at the starting dose level. Median age was
80 years (range 50-89). 7 patients had a luminal disease while 3 patients had
an HER2 positive disease. No patient suspended current systemic treatment. No
protocol defined DLTs were observed. When examining AEs, grade 2 skin toxicity occurred in 4 patients
with diseases located next to the skin and showing clinical retraction. All 10 patients, with a minimum follow-up of 6
months, were evaluable for response: 3
achieved a complete response, 6 achieved a partial response and 1 showed a
stable disease with a clinical benefit (resolution of skin retraction). The mean reduction
in the sum of the largest diameters of target lesions was of 61.4% (DS=17.0%).
In all patients with a skin retraction, retraction disappeared after treatment.
Conclusion
SABR
to primary breast cancer seems feasible and associated with symptoms reduction.
Continued accrual to this study is needed to confirm the safety and assess the
MTD.