Radiotherapy for Ledderhose disease: a randomised, double-blind, phase III trial (NCT03507010)
Anneke de Haan,
The Netherlands
OC-0834
Abstract
Radiotherapy for Ledderhose disease: a randomised, double-blind, phase III trial (NCT03507010)
Authors: Anneke de Haan1, Johanna G.H. van Nes2, Peter-Paul van der Toorn3, A. Helen Westenberg4, M. Willemijn Kolff5, Hans Paul van der Laan1, Paul M.N. Werker6, Johannes A. Langendijk1, Roel J.H.M. Steenbakkers1
1University Medical Center Groningen, Radiation Oncology, Groningen, The Netherlands; 2Radiotherapeutisch Instituut Friesland, Radiation Oncology, Leeuwarden, The Netherlands; 3Catharina Hospital, Radiation Oncology, Eindhoven, The Netherlands; 4Radiotherapiegroep, Radiation Oncology, Arnhem, The Netherlands; 5Amsterdam University Medical Center, Radiation Oncology, Amsterdam, The Netherlands; 6University Medical Center Groningen, Plastic Surgery, Groningen, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Radiotherapy
is considered a treatment option for Ledderhose disease. However, the added
value has never been confirmed in a randomised controlled trial (RCT). Therefore,
the main purpose of this study was to investigate the benefit of radiotherapy for
Ledderhose disease in a RCT.
Material and Methods
The
LedRad-study is a prospective, multicenter, randomised, double-blind, phase III
trial comparing radiotherapy to sham-radiotherapy (placebo), as treatment for
patients with symptomatic Ledderhose disease. The main inclusion criteria were;
pain from Ledderhose disease ≥ 2 on the numeric rating scale (NRS), no previous
surgery and/or radiotherapy to the affected foot and age ≥ 18 years. Age and
gender were used as stratification factors. Radiotherapy consisted of 10
fractions of 3 Gy administered in two separate courses of five daily fractions,
with an interval of 10 weeks. Procedures in both groups were identical, except that
treatment delivery was simulated for the patients randomised to receive sham-radiotherapy.
Unblinding of the treatment was performed 18 months after the end of
(sham-)radiotherapy. The primary endpoint was the pain score at 12 months after
treatment, measured with the NRS. Secondary endpoints were the pain score at 6 and
18 months after treatment, quality of life (QoL), walking abilities, changes in
nodule size, cost-effectiveness and safety/toxicity.
Results
From
January 2018 to October 2019, 84 patients (27 men and 57 women) were included
in the study. Mean age at time of inclusion was 56 years (range 19 to 75 years).
A total number of 130 feet were treated; 65 in the radiotherapy group and 65 in
the sham-radiotherapy group, respectively. At 12 and 18 months after treatment,
patients from the radiotherapy group had a statistically significant lower mean
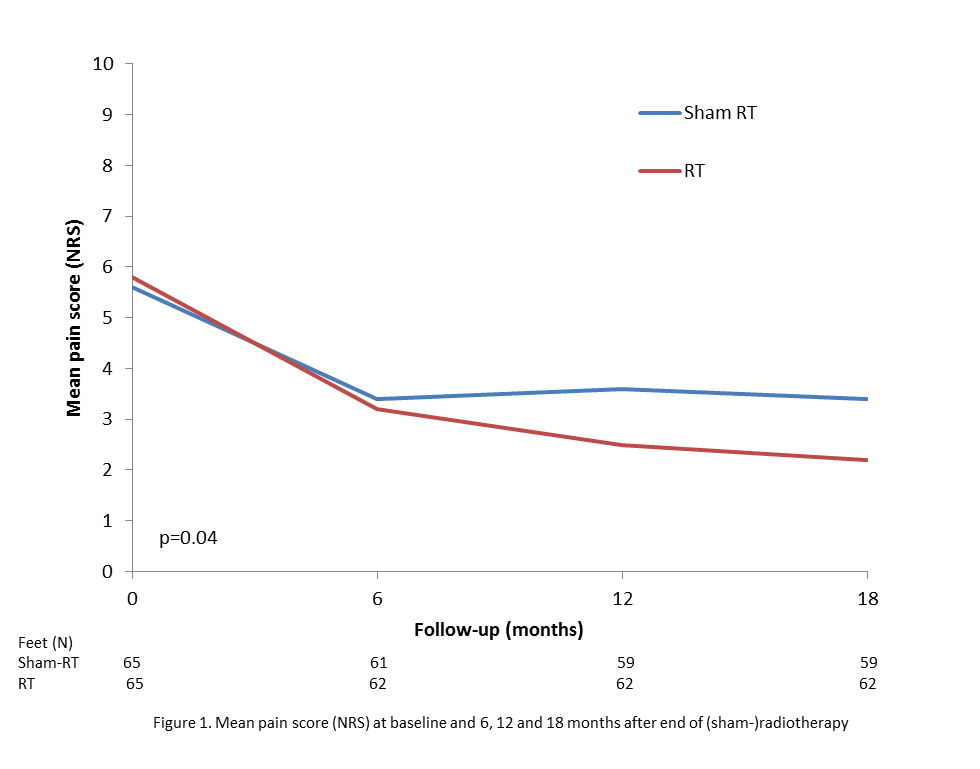
pain score compared to patients in the sham-radiotherapy group (2.5 vs. 3.6 and
2.2 vs. 3.4 respectively, figure 1). Pain response at 12 months was 74% for the
radiotherapy group and 56% for the sham-radiotherapy group. Twelve months after
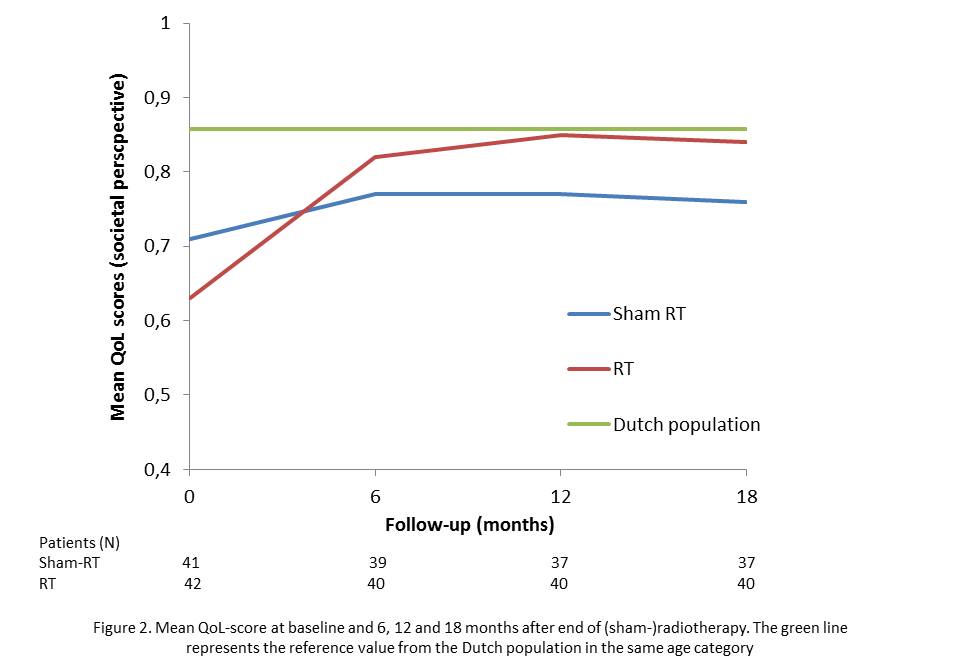
the end of study-treatment QoL scores of the radiotherapy group improved and
were comparable to the score of the Dutch general population in the same age
category (figure 2). Multilevel testing for QoL scores showed significantly higher
QoL scores for the radiotherapy group compared to the sham-radiotherapy group
(p<0.001). Also patients from the radiotherapy group had a significantly
higher mean walking speed and step rate when walking fast on bare feet (p=0.02).
Conclusion
This
study showed that radiotherapy for symptomatic Ledderhose disease results in
pain reduction, improvement of QoL scores and improvement of walking abilities.
The observation of the large placebo effect in the sham-radiotherapy group is yet
unclear and needs further investigation.