Anatomical evaluation of the breast treatment planning strategy for the randomised DBCG proton trial
Maria Fuglsang Jensen,
Denmark
PH-0549
Abstract
Anatomical evaluation of the breast treatment planning strategy for the randomised DBCG proton trial
Authors: Maria Fuglsang Jensen1, Line Bjerregaard Stick1, Ihsan Bahij1, Mette Giørtz1, Morten Høyer1, Camilla Jensenius Skovhus Kronborg1, Ebbe Laugaard Lorenzen2, Hanna Rahbek Mortensen1, Petra Witt Nyström1, Stine Elleberg Petersen1, Rikke Lysemose Poulsen1, Pia Randers1, Linh My Hoang Thai1, Esben Svitzer Yates3, Birgitte Vrou Offersen1,3
1Aarhus University Hospital, Danish Centre for Particle Therapy, Aarhus, Denmark; 2Odense University Hospital, Laboratory of Radiation Physics, Odense, Denmark; 3Aarhus University Hospital, Department of Oncology, Aarhus, Denmark
Show Affiliations
Hide Affiliations
Purpose or Objective
Proton
pencil beam therapy for selected breast cancer patients provides an alternative
to photon therapy, as the dose to heart and lungs can be reduced without compromising
target coverage. Robustness towards inter-fractional changes, however, pose an
issue for proton therapy. Changes due to the breast mobility or physiological swelling
or shrinkage challenge the treatment quality. We investigate the anatomical robustness
of the initial 20 patients with early breast cancer treated at the Danish
Centre for Particle Therapy (DCPT). The patients are pilots to the Danish
Breast Cancer Group (DBCG) proton trial (NCT04291378) and selected based on high
photon dose to the heart or lung.
Material and Methods
The
DCPT treatment strategy is free-breathing, 2-3 en face fields combined
with single field- and robust optimization, a 5 cm range shifter and a 5 mm distal
margin to all CTVs optimized with low priority. The CTV consists of: CTVp
breast/chest wall, CTVn levels 2-4, interpectoral nodes, internal mammary nodes
(IMN) and for 13/20 patients CTVn level 1. The prescribed dose is 50 Gy(RBE) in
25 fractions (12/20) or 40 Gy(RBE) in 15 fractions (8/20). 5/20 received
simultaneously integrated boost. The planning objectives are V95%>98% for
CTVp and V90%>98% for CTVn. A robust evaluation is performed using 14 combined
scenarios (table 1b) requiring a worst-case V95%>95% for CTVp and
V90%>95% CTVn. 2-3 scenarios with lower CTVn IMN coverage are allowed in
order to minimize the heart dose. A final anatomical evaluation is performed using
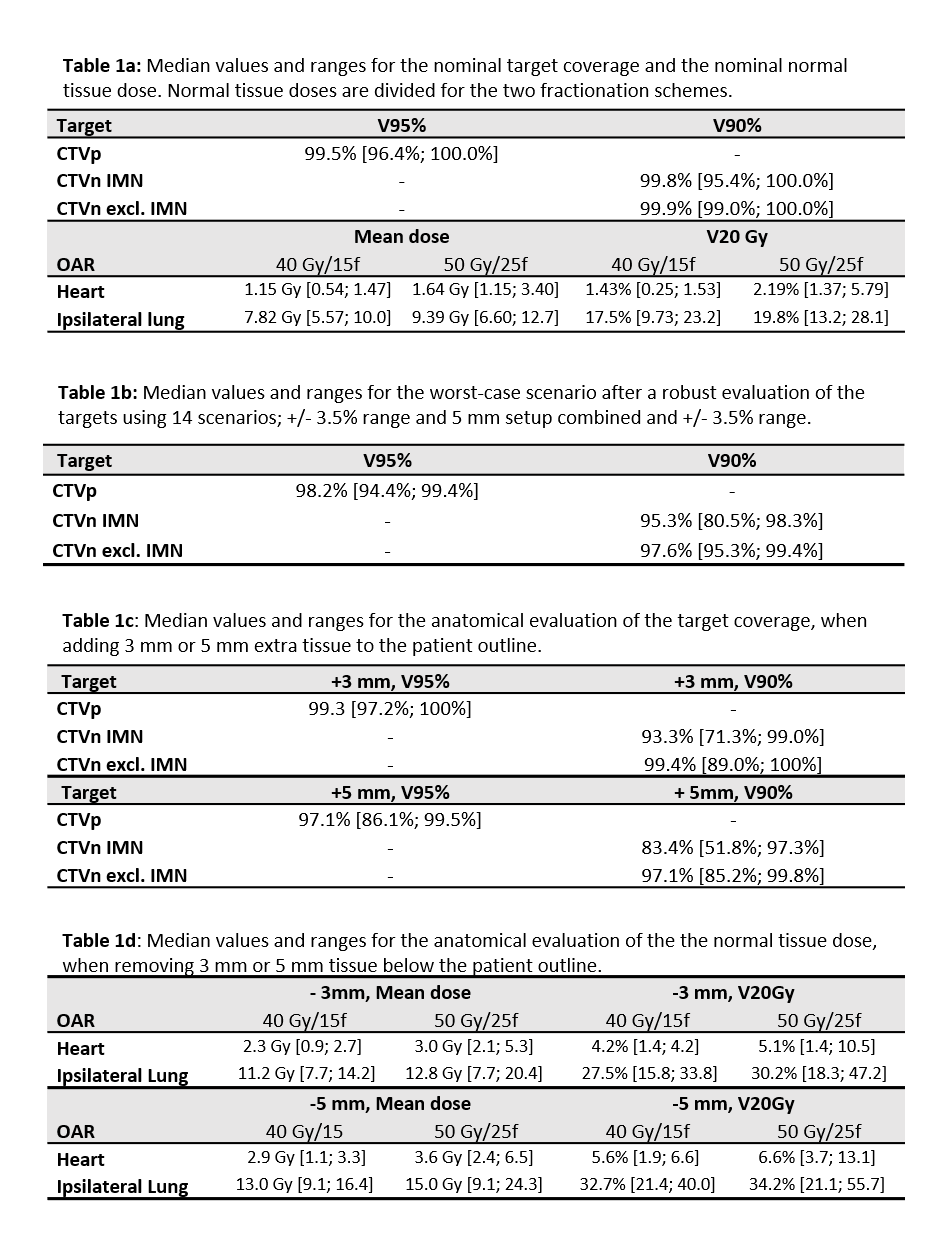
4 artificial CTs created by adding/removing 3 mm and 5 mm tissue to/from the
patient outline, figure 1a.
Results
The
target coverage, normal tissue dose, robust and anatomical evaluations are shown
in table 1. All plans meet the nominal and robust requirements. The anatomical
evaluations show that 3 mm shrinkage approximately doubles the mean heart dose
and 5 mm swelling reduces the nominal CTVn IMN coverage below the constraints. Figure
1b illustrates the steep dose fall-off towards the heart. These results are
used to create a patient specific variable "outer limit" tolerance structure
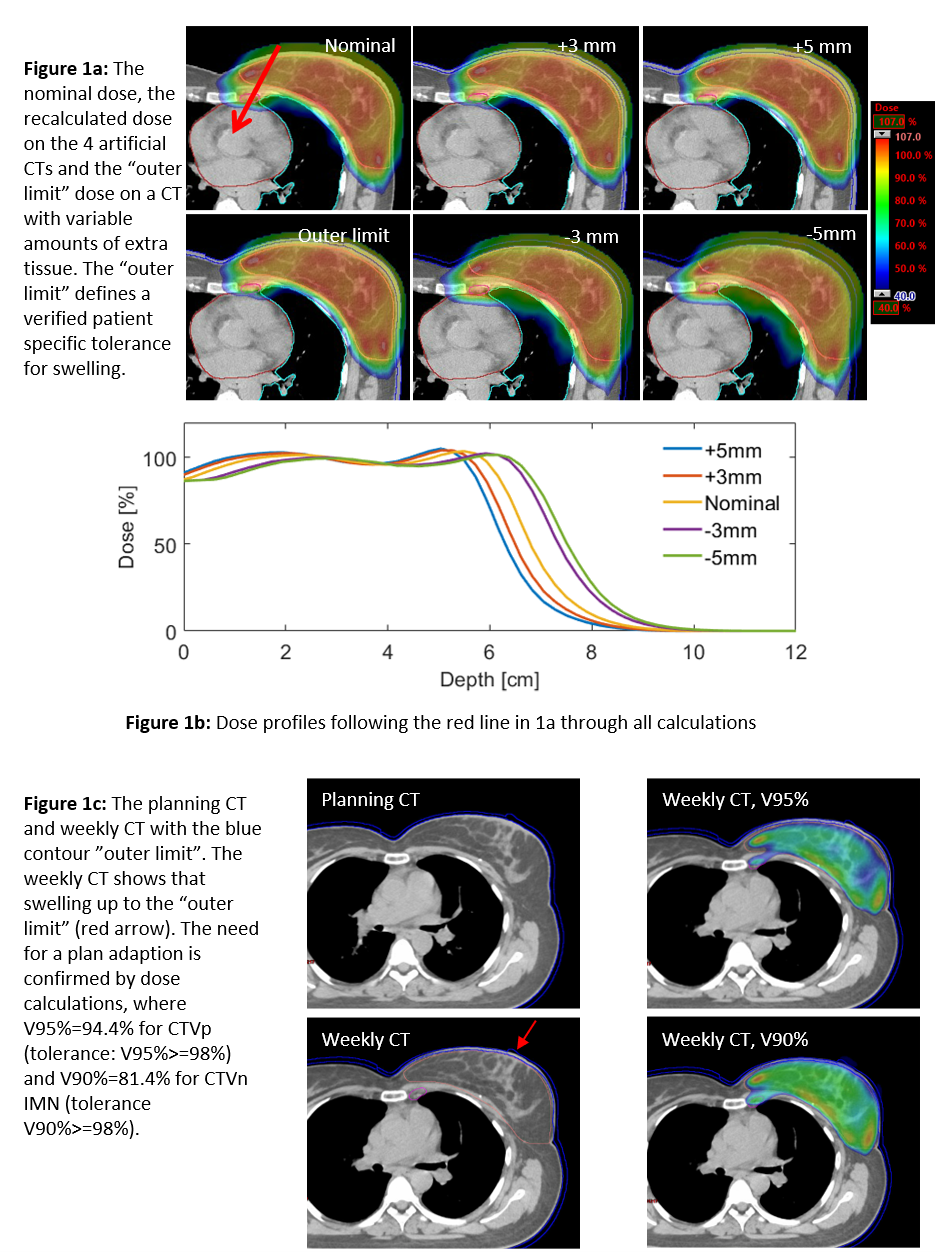
to be used during daily, online CBCT evaluations. Based on the weekly CT scans,
a total of 15 plan adaptions were performed: 4/15 due to shrinkage and 9/15 due
to extra tissue. Figure 1c shows an example of a plan adaption due to 5 mm swelling.
Conclusion
Robustness
towards anatomical changes must be evaluated separately from range and setup
uncertainties. Small amounts of extra tissue deteriorate the target coverage, while
shrinkage increases the normal tissue dose. Using this planning strategy, the CTVns
are in general robust to 3 mm and the CTVp to 5 mm extra tissue. Increasing it
further will have consequences for the heart and lung dose. At DCPT, the weekly
CT scans are now replaced by an adaptive re-scanning strategy based purely on daily
CBCT evaluations using the variable outer limit as tolerance combined with a 3
mm tolerance on shrinkage.