Neural network based synthetic CTs for adaptive proton therapy of lung cancer
Adrian Thummerer,
Germany
OC-0478
Abstract
Neural network based synthetic CTs for adaptive proton therapy of lung cancer
Authors: Adrian Thummerer1, Paolo Zaffino2, Carmen Seller Oria1, Arturs Meijers1, Gabriel Guterres Marmitt1, Joao Seco3,4, Johannes Langendijk1, Antje Knopf1, Antje Knopf5, Maria Francesca Spadea2, Stefan Both1
1University Medical Center Groningen, Department of Radiation Oncology, Groningen, The Netherlands; 2Magna Graecia University Catanzaro, Department of Experimental and Clinical Medicine, Catanzaro, Italy; 3Deutsches Krebsforschungszentrum (DKFZ), Department of Biomedical Physics in Radiation Oncology, Heidelberg, Germany; 4Heidelberg University, Department of Physics and Astronomy, Heidelberg, Germany; 5University Hospital of Cologne, Department I of Internal Medicine, Center for Integrated Oncology Cologne, Cologne, Germany
Show Affiliations
Hide Affiliations
Purpose or Objective
Adaptive proton
therapy (APT) accounts for anatomical and physiological changes to ensure
target coverage and organ-at-risk sparing during the entire treatment course. An
imaging modality that may detect these anatomical changes is cone-beam computed
tomography (CBCT). However, CBCT-images suffer from severe image artifacts
(e.g. scatter) that hinder accurate proton dose calculations. Deep
convolutional neural networks (DCNN) have shown potential to correct CBCTs and
create synthetic CTs (sCTs) that enable proton dose calculations in various
anatomical locations (e.g head&neck, pelvis). In this study such a DCNN together with an accompanying
planning CT (pCT) based patient specific correction technique was used to
generate sCTs and their suitability for adaptive proton therapy of lung cancer
patients was evaluated in terms of image quality and dosimetric accuracy.
Material and Methods
A dataset consisting of CBCT- and same-day repeat
CT-images from 33 lung cancer patients, treated with proton therapy, was used
to train and evaluate the DCNN. 3-fold cross validation was employed to utilize
all 33 patients for image and dosimetric evaluation. After the DCNN-conversion,
an automatic patient specific correction method, using a smoothed and truncated
difference map between the pCT and sCT, was introduced, mainly to correct CT-numbers
of lung tissue, which are difficult to generate consistently and accurately by
the DCNN.
For image quality
assessment, mean absolute error (MAE) and mean error (ME) were calculated for
sCTs with (sCTcor) and without (sCTorig) the pCT-based
correction method. For the dosimetric evaluation, clinical treatment plans were
recalculated on both synthetic CTs and gamma pass ratios (3%/3mm) were used to
compare dose distributions to those calculated on the reference CT scans.
Results
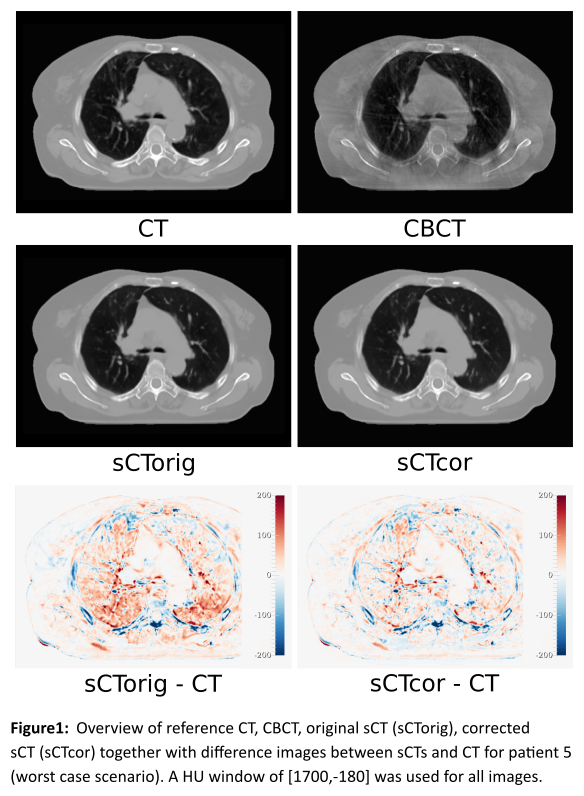
Figure 1 shows an
overview of CBCT, CT, sCTorig and sCTcor for patient 5
together with difference images between sCTs and CT. Average MAEs (ME) of 34.7±7.2
HU (5.2±9.8 HU) and 30.8±4.7 (2.7±4.8 HU) were observed for sCTorig and
sCTcor respectively. The recalculation of clinical treatment plans
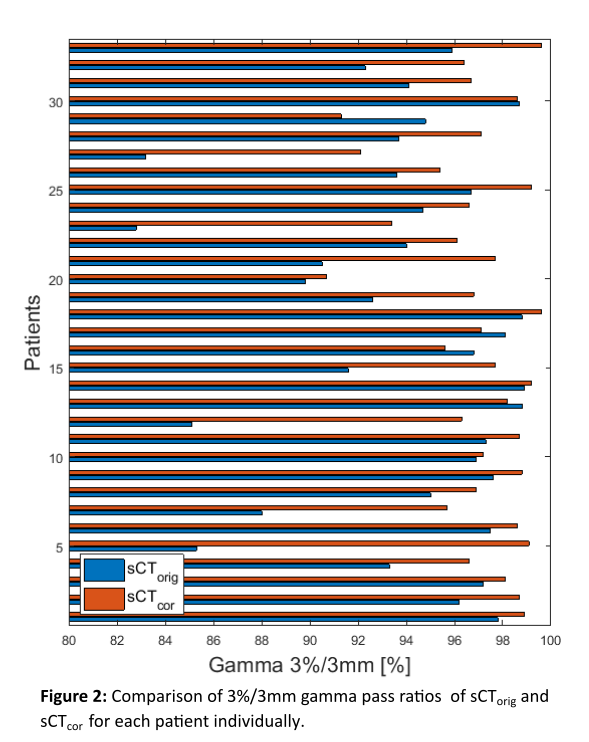
resulted in average gamma pass ratios of 93.9±4.6 % for sCTorig and
96.9±2.2 % for sCTcor. Results from the gamma analysis are presented
in Figure 2 for each patient individually.
Conclusion
The image quality
evaluation and the dosimetric accuracy assessment indicates that neural network
based sCTs combined with a patient specific correction method may be utilized for
adaptive proton therapy in lung cancer patients.