52 patients with a total of 105 liver metastases were treated with SBRT since June 2015 to October 2018. Median age was 69.7 years (36-90 years), 34 were men (65.4%) and 18 women (34.6%). Primary tumor was colorectal 38(73.1%) followed by lung 5(9.6%), breast 4(7,7%) and pancreas 3(5.8%). Liver metastases were synchronous in 24p (46.2%) and metachronous in 28(53.8%). Other oligo-extrahepatic, previously treated, metastases, were present in 21p (40.4%). Previous local treatments were performed in 19p (36.5%) and previous chemotherapy was administered in 48p (92.3%), range:1-4 lines.
Prior to treatment, internal fiducial markers were placed in 34p (65.4%). GTV was based on CT images with i.v. contrast. ITV was created with 4DCT when no respiratory Gating was performed. PTV was GTV or ITV + 5 mm margin. Median GTV diameter was 2.6 cm (0-9-6.7 cm) median PTV volume was 7.5 cc (range 0.4–150.5 cc). 23p (44%) were treated of a single liver metastases, 14 p (27%) of 2 lesions and 15p (29%) of 3 or more (range 3-8), 15 patients received more than 1 course of SBRT after out-field intrahepatic recurrence median 2 (range 2-4).
Prescription dose to 95% of PTV was 60Gy (3x20Gy) in 45 lesions (42.8%), 50Gy (5x10Gy) in 34 (32,3%), 45Gy (3x15Gy) in 34 (32.3%) and 54Gy (3x18Gy) in 1 (0.95%). BED10Gy was ≥100Gy for 100% of the lesions. IMRT/VMAT was performed. Daily IGRT was mandatory. Respiratory control with Exactrac Adaptive Gating (10p) or Active Breathing Coordinator (14p) was performed in 24p (46%) and in 28p (64%) a Dampening technique was used.
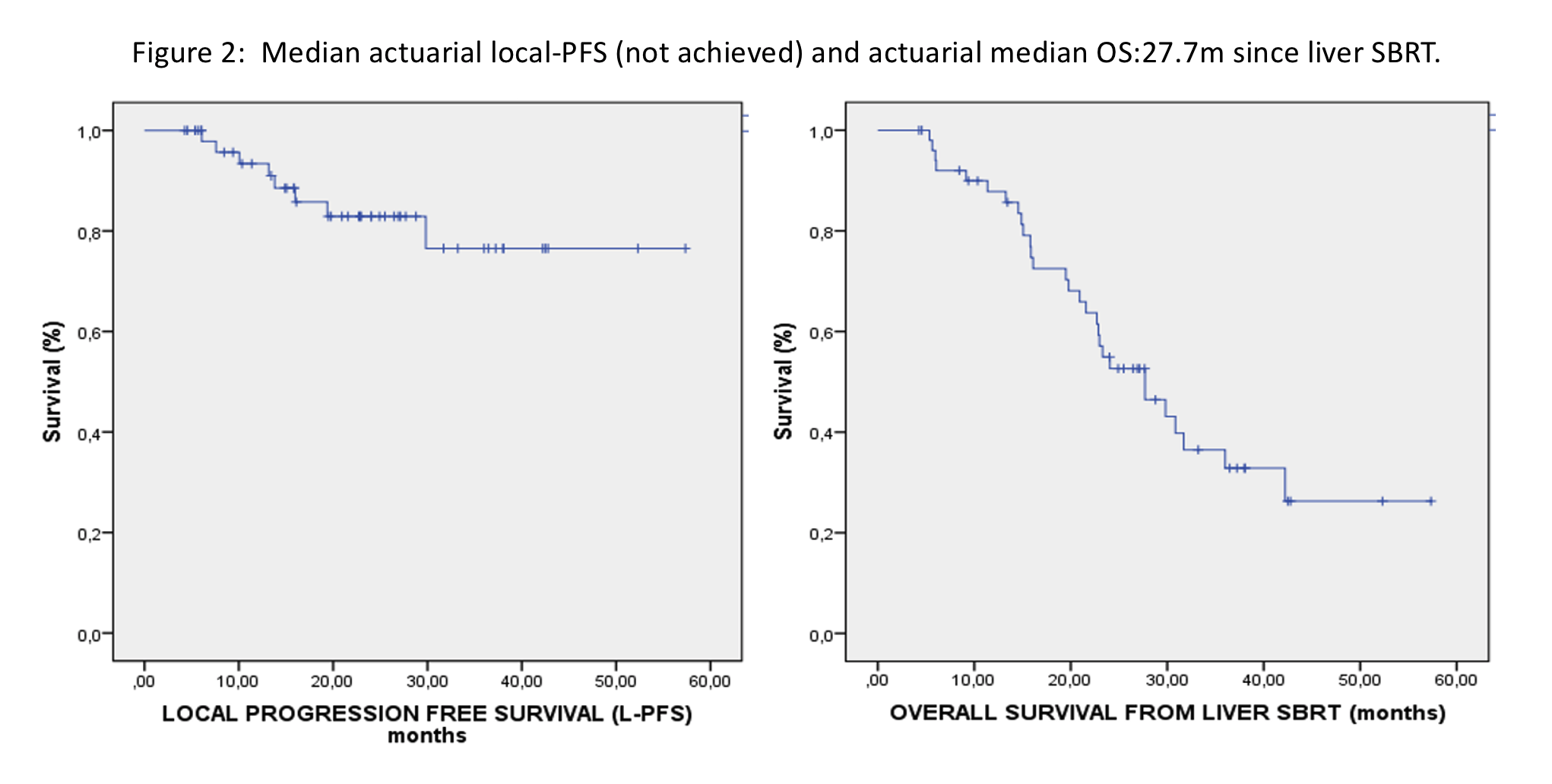
With a median follow-up of 23.1 months (m), median actuarial local progression-free survival (PFS) was not achieved. At the time of analysis, 29 patients are still alive (55.8%). The actuarial local-PFS was 82.9% at 24m and 76.5% at 36m. The actuarial median liver-PFS and distant-PFS was 11.0 and 10.8m respectively. The actuarial median overall survival was 27.7m since liver SBRT and 52.5m since liver metastases diagnosis. Grade 1-2 toxicity was observed in 31p (59,6%): 15p (28.8%) had transitory blood test alterations and 8p (15.4%) asthenia. No acute or late toxicities grade ≥3 was observed.