Brachytherapy TG-43 dosimetry characterization of the INTRABEAM source
OC-0132
Abstract
Brachytherapy TG-43 dosimetry characterization of the INTRABEAM source
Authors: David Santiago Ayala Alvarez1, Peter G F Watson1, Marija Popovic1, Veng Jean Heng1, Michael D C Evans1, Jan Seuntjens1
1McGill University, Medical Physics Unit, Montreal, Canada
Show Affiliations
Hide Affiliations
Purpose or Objective
The
INTRABEAM system (Carl Zeiss Meditec AG) is an electronic brachytherapy device
designed for intraoperative radiotherapy (IORT) applications. Despite its
benefits and extended use for common diseases as brain and breast cancers, the
INTRABEAM x-ray source has not been characterized according to the AAPM TG-43
specifications for brachytherapy sources. This restricts its modeling in commercial treatment planning systems
(TPSs), with the consequence that the doses to organs at risk (OARs) are
unknown. Knowledge of these doses is typically important when dose
distributions need to be compared and combined with external beam dose
distributions. The aim of this work is to characterize the INTRABEAM source
according to the TG-43 brachytherapy dosimetry protocol.
Material and Methods
The dose distribution in water around the
INTRABEAM source was determined with Monte Carlo (MC) calculations using
egs_brachy, a user code of EGSnrc. MC statistical uncertainties were in the
range 0.1% to 0.4% at 1 to 5 cm from the source tip in its longitudinal axis.
For the validation of the MC model, depth dose calculations in water along the
source longitudinal axis were compared with measurements in two different
setups: (1) using a water phantom provided by the source manufacturer and a
soft x-ray ionization chamber (PTW 34013) and (2) with a customized setup using
a Wellhöfer water tank and synthetic diamond detectors (microDiamond PTW
TN60019), with low volume averaging effects and uncertainties from the detector
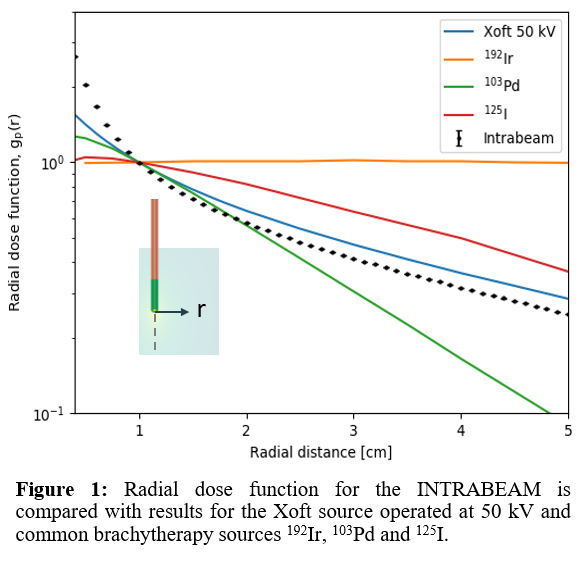
geometry. The calculated radial dose function for the INTRABEAM is compared
with published data for the Xoft Axxent® (a subsidiary of iCAD, Inc. Nashua,
NH) source, and several commonly used HDR and LDR brachytherapy sources.
Results
Measurements in water with the ionization
chamber agreed with the MC model calculations within uncertainties. These
combined uncertainties vary with depth in water and have an approximate value
of 3.1% at 1 cm from the source tip. The use of the microDiamond yielded local
percent differences within uncertainties in points of steeper dose gradients.
The radial dose function (Figure 1) presents a steep fall-off close to the
INTRABEAM source (< 1 cm) with a gradient higher than that of conventional
brachytherapy radionuclides (192Ir, 103Pd, and 125I),
but it is partially flattened at larger distances with a similar fall-off as
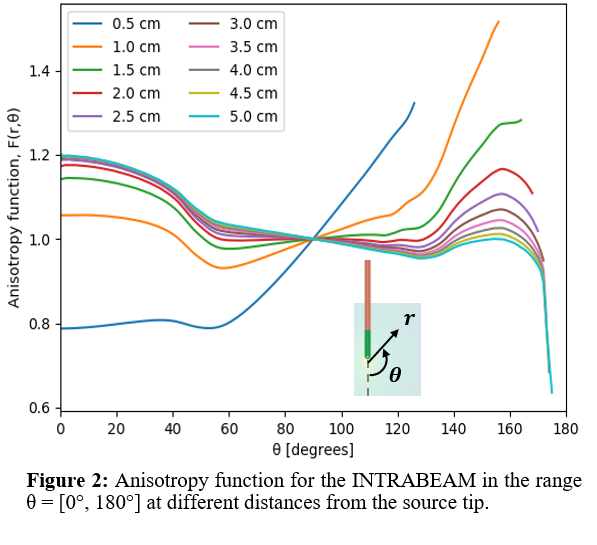
the Xoft source. The simulated 2D anisotropy values (Figure 2) were mainly
uniform along θ = 0° for r > 1 cm, and gradually decreased towards θ ≈ 120°.
For regions close to the source, the behavior was strongly affected by the beam
attenuation in the elements of the source walls.
Conclusion
This work presents the MC calculated TG-43 parameters for
the INTRABEAM, which constitute the necessary data required by conventional
brachytherapy TPSs. In the proximity of the source, the dose distribution
exhibits a higher gradient than other sources and the 2D anisotropy function is
strongly affected by the wall materials.