Neoadjuvant chemotherapy may alter extranodal extension in head and neck squamous cell cancers
PO-0125
Abstract
Neoadjuvant chemotherapy may alter extranodal extension in head and neck squamous cell cancers
Authors: Deep Chakrabarti1, Mranalini Verma1, Sumaira Qayoom2, Divya Kukreja1, Shiv Rajan3, Naseem Akhtar3, Vijay Kumar3, Kirti Srivastava1, Rajeev Gupta1, Madan Lal Bhatt1
1King George's Medical University, Radiotherapy, Lucknow, India; 2King George's Medical University, Pathology, Lucknow, India; 3King George's Medical University, Surgical Oncology, Lucknow, India
Show Affiliations
Hide Affiliations
Purpose or Objective
Extranodal extension(ENE) was incorporated in staging head and neck squamous cell carcinoma(HNSCC) in 2018. The interpretation of pathological ENE, a key indicator of postoperative chemoradiotherapy, is unknown in resected HNSCC patients who have received prior neoadjuvant chemotherapy(NACT). We wanted to investigate whether NACT alters ENE in a subset of patients who are clinically ENE-positive but may turn out to be ENE-negative post-NACT on the resection specimen.
Material and Methods
This retrospective cohort study included patients with previously untreated, resectable or borderline resectable, non-metastatic HNSCC treated with definitive intent by a combined team approach who underwent surgery as part of their treatment between January and December 2020 at a tertiary care academic university hospital in India. They had to be fit for receiving multimodality treatment. Eligible patients underwent upfront surgery or NACT followed by surgery as per institutional protocols, followed by postoperative radiotherapy as per the relevant indications.
Results
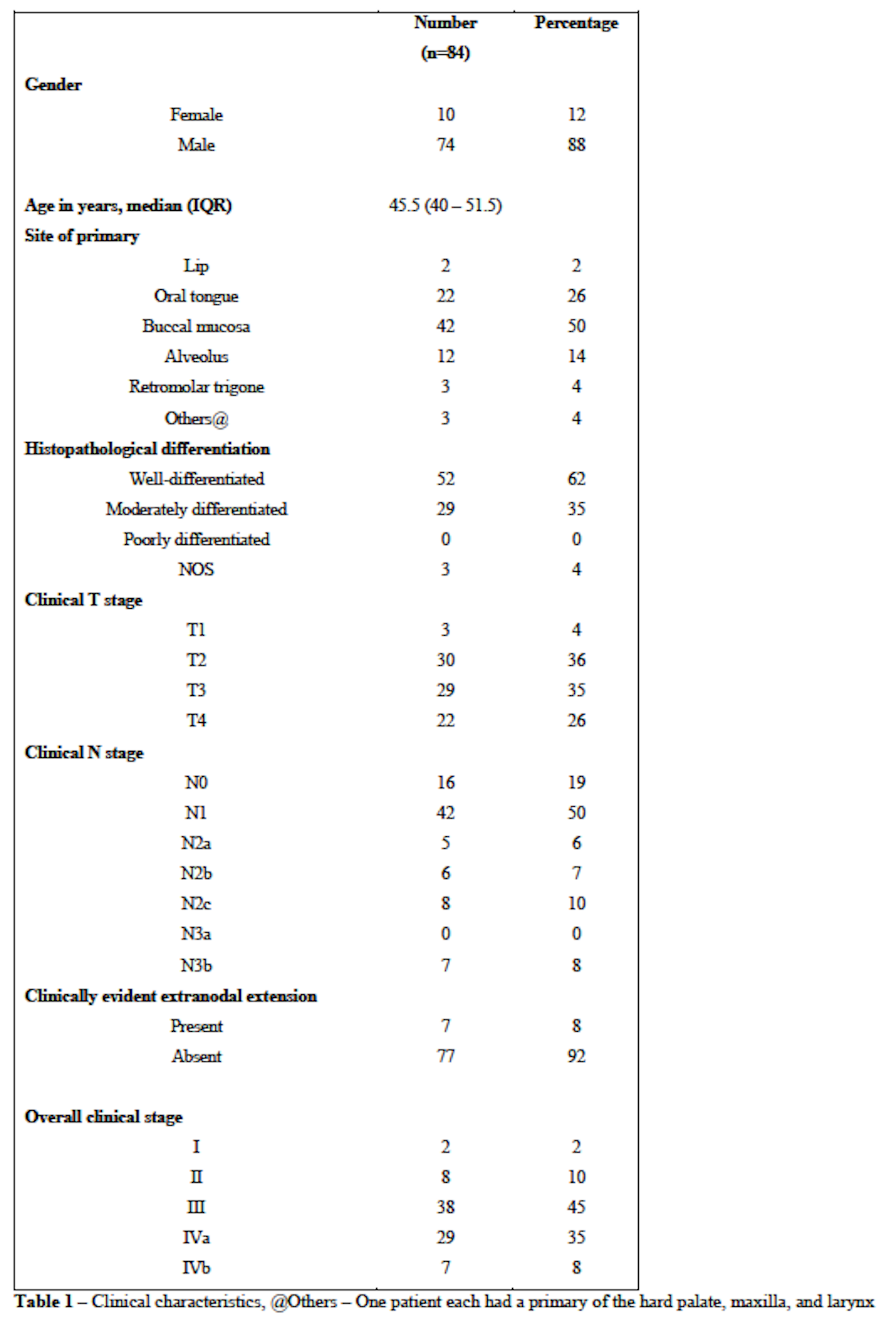
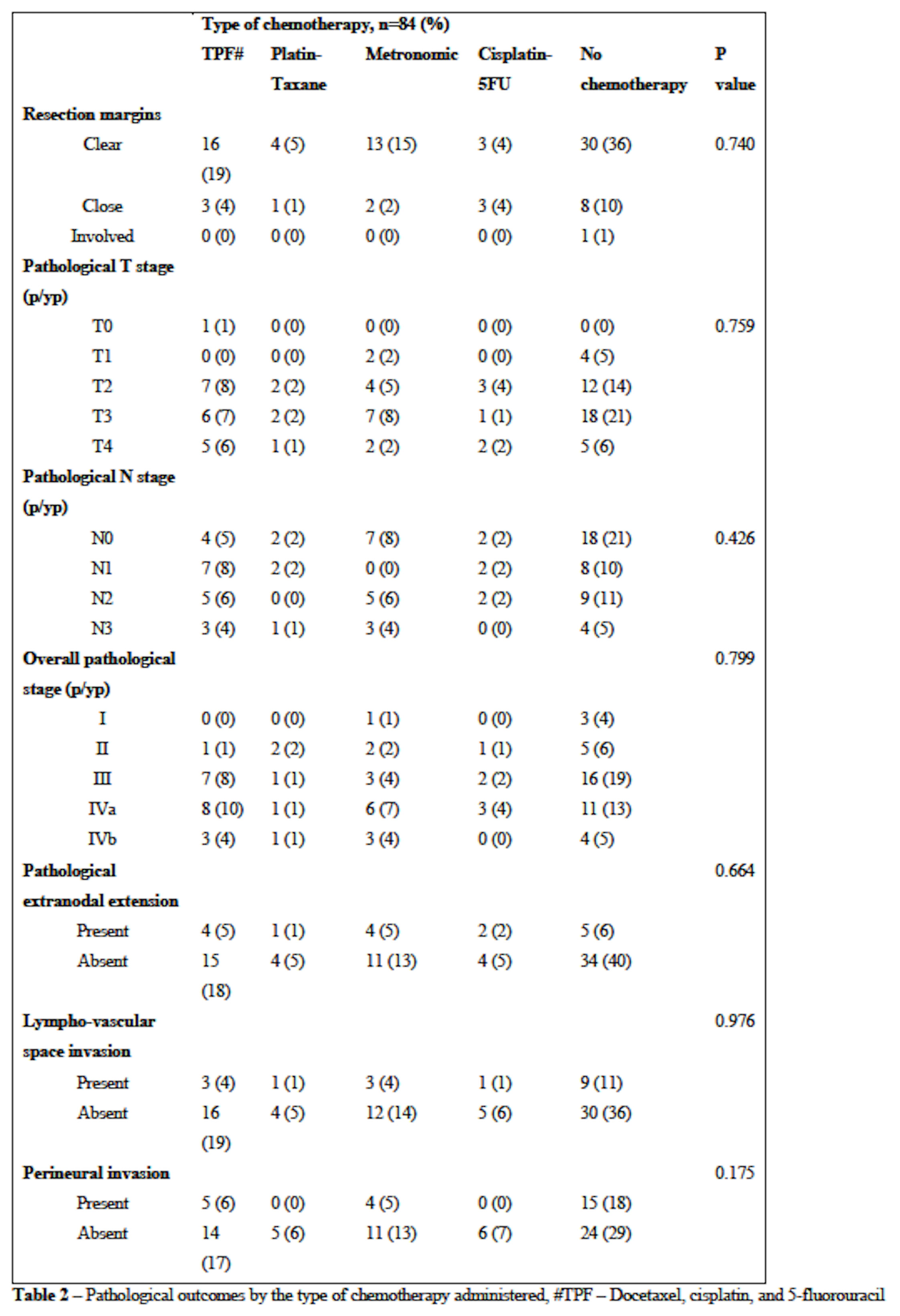
Between January to December 2020, 84 patients underwent curative-intent treatment after a joint team decision. Most had a primary in the buccal mucosa(50%) or tongue(26%), were T3/T4(61%), node-positive(81%), with a composite stage of III or IV(88%). Forty-five patients(54%) received NACT. Sixty-seven patients(80%) underwent postoperative radiotherapy, of which 20(24%) received chemoradiotherapy. There were no significant differences in the resection margin(P=0.740), pathological T stage(P=0.759), pathological N stage(P=0.426), overall stage(P=0.799), and pathological ENE(P=0.664) between the different chemotherapy regimens. There was no significant association between clinical ENE and pathological ENE in patients undergoing NACT(P=0.313). Five patients(11%) who were clinically ENE-positive became ENE-negative after receiving NACT.
Conclusion
Our study identified a sub-group of patients with clinically-evident ENE who may become ENE-negative due to the effects of NACT. Future research may be undertaken to identify if concurrent chemotherapy can be avoided in these patients when they receive radiotherapy, and a randomized clinical trial may be designed for the same.