MR-guided SBRT boost for patients with recurrent gynecological cancers ineligible for brachytherapy
PO-1311
Abstract
MR-guided SBRT boost for patients with recurrent gynecological cancers ineligible for brachytherapy
Authors: Indrawati Hadi1, Rieke von Bestenbostel1, Stephan Schönecker1, Katrin Straub1, Lukas Nierer1, Raphael Bodensohn1, Michael Reiner1, Guillaume Landry1, Claus Belka1,2, Maximilian Niyazi1,2, Stefanie Corradini1
1LMU Munich University Hospital, Radiation Oncology, Munich, Germany; 2German Cancer Consortium (DKTK), Radiation Oncology, Munich, Germany
Show Affiliations
Hide Affiliations
Purpose or Objective
External beam radiotherapy (EBRT) with concurrent
chemotherapy followed by a brachytherapy (BT) boost is the standard of care for
most patients with locally advanced or recurrent gynecological cancer
(LA(R)GC). However, not every patient is suitable for a BT boost due to the
extent or localization of the tumor. Therefore, we investigated the feasibility
of an MR-guided SBRT boost (MR-SBRT-boost) following EBRT.
Material and Methods
Patients with LA(R)GC treated at our institution with a
MR-SBRT-boost using a 0.35T hybrid MR-Linac (Viewray Inc., Mountain View, CA),
were retrospectively analyzed. The patients were not suitable for BT due extensive
infiltration of the pelvic wall (37.5%) and other adjacent organs (62.5%).
Online-adaptive treatment planning was performed to control for interfractional
anatomical changes. Treatment parameters and toxicity were evaluated to assess
the feasibility of MR-SBRT-boost.
Results
Eight patients with recurrent cervical cancer (n=5; 62.5%), locally
advanced cervical cancer and rectal infiltration (cT4) (n=1; 12.5%), as well as
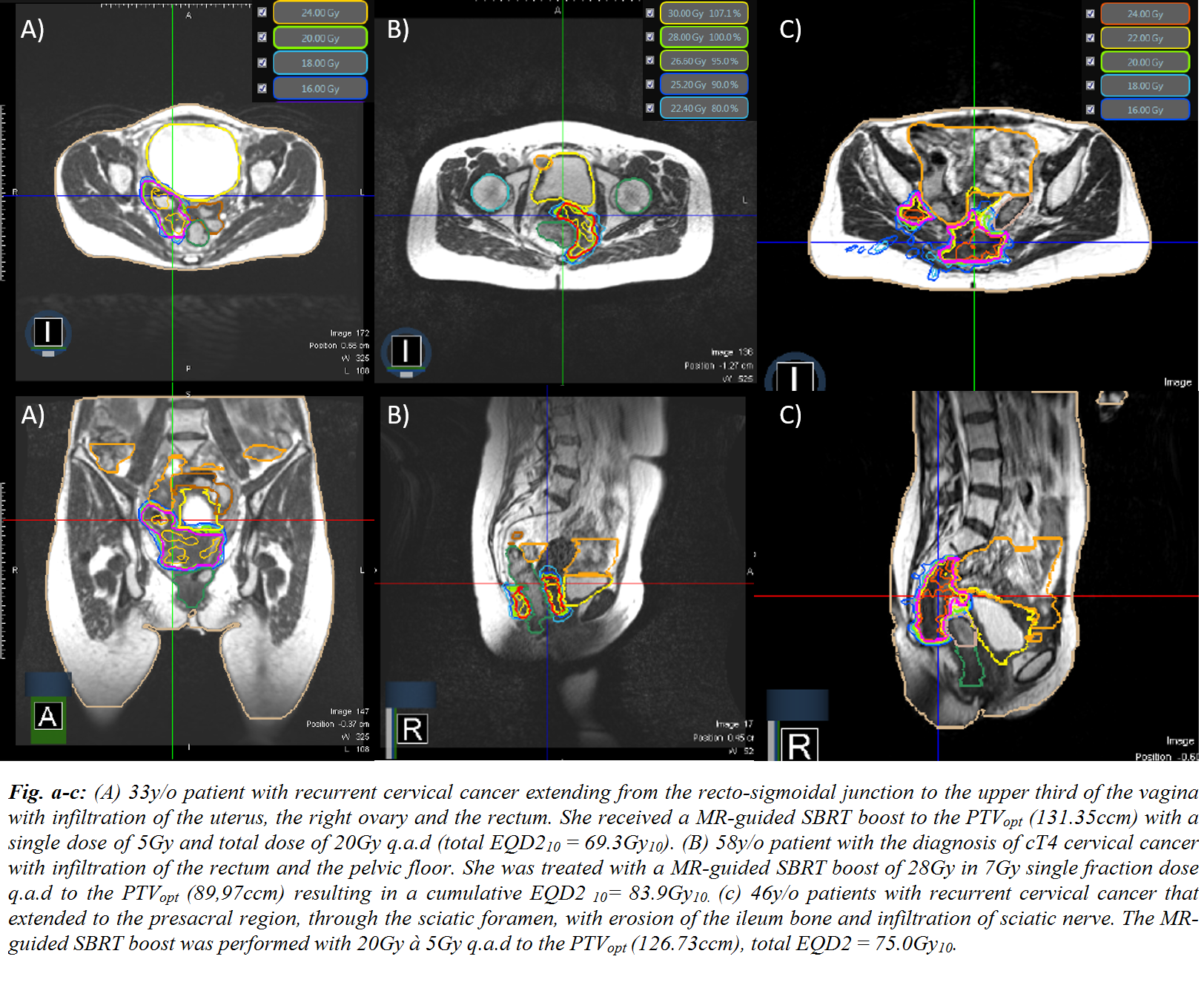
recurrent vaginal cancer (n=2; 25.0%) were treated between 03/2020 - 01/2021 (Fig. a-c). EBRT of the primary tumor
and the pelvic (62.5%)/paraaortic lymphatics (37.5%) was applied using a VMAT with
a median dose of 1.8Gy/fraction (fx) (range: 1.8 – 2.2Gy) to a median total
dose (TD) of 45.0Gy (45.0 – 55.0Gy). All patients received a concurrent
Cisplatin 40mg/m2 q1w. A simultaneous integrated boost (SIB) to positive
lymph nodes was given in 3 patients with a TD of 55-57.5Gy in 25 fx. The
MR-SBRT-boost was delivered every other day (q.a.d) with a median dose of 5.0Gy/fx
(4.0 – 7.0Gy) to a median TD of 20.0Gy (8.0 – 28.0Gy). The median HR-CTV was
28.9ccm (12.9 – 111.75ccm) and the median cumulative TD of the combined
EBRT+MR-boost of the HR-CTV was EQD210 71.3Gy (69.3 – 83.9Gy10). An optimized PTV (PTVopt) was obtained from subtracting organs at risk (OAR) with a
3mm isotropic expansion from the PTV. Median PTVopt was 43.5ccm (24.2 – 131.35ccm). Using OAR constraints of BT, a
cumulative median EQD23 for the rectum D2ccm was 63.7 Gy3 (51.5 – 72.6Gy3); bladder D2ccm 72.2Gy3 (67.1 – 83.6Gy3); sigmoid D2ccm 45.7Gy3 (43.2 – 58.7Gy3); bowel D2ccm 59Gy3 (47.7 – 70.0Gy3). The median net beam on time/fx was 5.9 minutes (1.53 –
11.67 min), and the median overall treatment time (OTT)/fx was 79 min (44.0 –
89.5 min), including the adaptive workflow in 100% of fractions. The median OTT
from the beginning of EBRT to the last SBRT fraction was 50 days (39 – 56
days). The most common side effects were diarrhea CTC°I-II (n= 4, 50.0%) and dysuria
CTC°I-II (n= 5; 62.5%). No CTCAE ≥ °III and no worsening of acute side effects
after the MR-SBRT-boost were observed.
Conclusion
These early results report the feasibility of an MR-guided
SBRT boost approach in patients with LA(R)GC who were not candidates for BT.
When classical BT-OAR constraints are followed, acute toxicity was favorable.
However, long-term follow-up is needed to validate the results.