Mean heart dose predicts survival of NSCLC patients treated with chemoradiotherapy and durvalumab
PO-1183
Abstract
Mean heart dose predicts survival of NSCLC patients treated with chemoradiotherapy and durvalumab
Authors: Eva Ćirić1, Staša Jelerčič1, Martina Vrankar1, Jasna But Hadžić1, Karmen Stanič1, Ana Lina Vodušek1
1Institute of Oncology Ljubljana, Radiation Oncology Department, Ljubljana, Slovenia
Show Affiliations
Hide Affiliations
Purpose or Objective
Since the results of PACIFIC trial, consolidation
treatment with durvalumab has become the standard of care for patients with
stage III unresectable non-small cell
lung cancer (NSCLC) with response to chemoradiotherapy (CRT). First
reports show comparable efficacy and toxicity of this treatment strategy in the
real-life setting. The data on factors predicting survival of these patients is
however scarce.
Material and Methods
In this retrospective analysis we reviewed patients with
stage III NSCLC and disease control after CRT who were included in durvalumab early
access programme in Slovenia. Survival curves were estimated using the
Kaplan-Meier method. Cox regression model was used for multivariate analysis to
evaluate the effect of different prognostic factors including radiotherapy
parameters.
Results
A total of 59 patients were included, median age 62 years
(36-73), 71.2% were male, 78.0% in stage IIIB or IIIC and 96.6% had ECOG 0-1.
66.1% had squamous-cell carcinoma and 30.5% adenocarcinoma. PD-L1 expression
was 0% in 22.0%, ≥1% in 66% and unknown in 11.9% of patients. Concurrent
chemotherapy was given to 59.3% of patients, sequential to 40.7%. Majority of
patients (86.4%) received a total dose of at least 60Gy. Median follow up
period was 28 months. Median post-CRT progression-free survival (PFS) for the
entire cohort was 22.6 months and not reached for overall survival (OS).
Estimated 12 and 18-month PFS rate were 67.8% and 55.9%, estimated 12 and
18-month OS rate were 84.7% and 72.8%. No patient experienced ≥G3 immune
related (IO) toxicity, whereas 47.5% patients experienced G2 or less IO
toxicity. Pneumonitis was observed in 9 patients (15.3%). There was no
significant difference in PFS or OS in patients with 0% vs ≥1% PD-L1 expression
and patients who received concurrent vs. sequential CRT. In the univariate
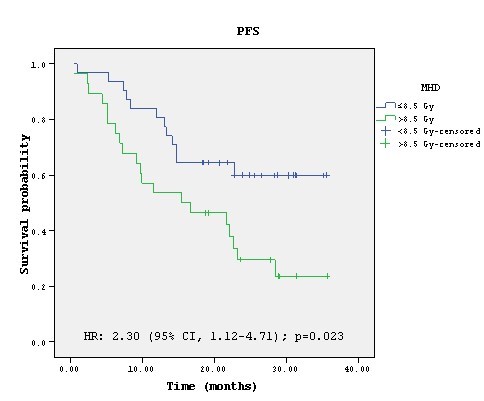
analysis mean heart dose (MHD) was correlated with significantly improved PFS
(p=0.023) and PTV volume showed trend toward significance (p=0.076). In the
multivariate analysis MHD remained significant with HR 2.11 (95% CI 1.02-4.37),
p=0.045. Estimated 12-month PFS rate for patients receiving MHD≤8.5Gy and
>8.5Gy was 80.6% and 53.6%.
Conclusion
Outcomes of patients in real-life setting are aligned
with PACIFIC trial. There might be an association between radiotherapy dose to
the heart and survival of patients treated with this combined therapy that
warrants further investigation.