Ultra-central lung tumors: safety and efficacy of protracted stereotactic body radiotherapy
Joyce Lodeweges,
The Netherlands
PO-1172
Abstract
Ultra-central lung tumors: safety and efficacy of protracted stereotactic body radiotherapy
Authors: Joyce Lodeweges1, Peter van Rossum1, Marcia Bartels2, Anne van Lindert3, Jacqueline Pomp2, Max Peters2, Joost Verhoeff1
1University Medical Center Utrecht, Radiation Oncology, Utrecht, The Netherlands; 2University Medical Center Utrecht, Radiation Oncology , Utrecht, The Netherlands; 3University Medical Center Utrecht, Pulmonology, Utrecht, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
For patients with early stage or medically inoperable
lung cancer, stereotactic body radiotherapy (SBRT) is a general accepted and
effective treatment option. While most successful data come from peripherally
located tumors, the role of SBRT in ultra-central tumors remains controversial.
The aim
of this single-center cohort study was to evaluate the safety and efficacy of
protracted SBRT with 60 Gy in 12 fractions (with a biological effective dose [BED10] of 90Gy) for
patients with ultra-central lung tumors.
Material and Methods
Patients with ultra-central lung tumors treated in our
institution with 60 Gy in 12 fractions from January 2012 until
April 2020 were included.
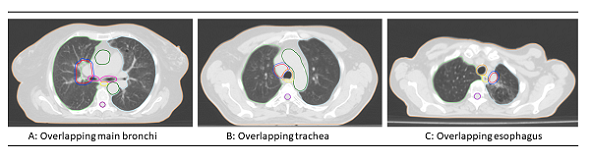
Ultra-central tumors were defined as planning target volume (PTV) abutting or
overlapping the main bronchi, trachea and/or esophagus (Figure). Data regarding patient-, tumor-, and
treatment-related characteristics were evaluated.
Results
A total of 72 patients met the criteria for
ultra-central tumor location. The
PTV abutted the main bronchus, trachea or esophagus in 78%, 21% and 21% of
cases, respectively. At a median follow-up of 19 months, 1- and 2-year local
failure-free survival rates were 98% and 85%, respectively. Overall survival
rates at 1 and 2 years were 77% and 52%, respectively. Grade 3 or higher
toxicity was observed in 21%, of which 10 patients (14% of total) died of
bronchopulmonary hemorrhage. A
significant difference between patients with or without grade ≥3 toxicity was
found for the mean dose (Dmean) to the main bronchus (p=0.015),
where a Dmean BED3 of ≥90 Gy increased the risk of grade
≥3 toxicity significantly. Age, tumor histology and antithrombotic therapy was
not significantly associated with the rate of grade ≥3 toxicity.
Conclusion
A
protracted SBRT regimen of 60 Gy in 12 fractions for ultra-central lung tumors
leads to high local control rates with acceptable toxicity in most patients,
albeit at the risk of serious toxicity and even mortality. Therefore, possible
risk factors of lung hemorrhage such as dose to the main bronchus, peri- or
endobronchial tumor location and anti–vascular endothelial growth factor
(anti-VEGF) or antithrombotic therapy should be taken into account. This study
suggests to limit the Dmean BED3 to the main bronchus to 90
Gy.