PSA BOUNCE AND BIOCHEMICAL FAILURE ANALYSIS IN LOW DOSE RATE IODINE-125 PROSTATE BRACHYTHERAPY BOOST
PO-1385
Abstract
PSA BOUNCE AND BIOCHEMICAL FAILURE ANALYSIS IN LOW DOSE RATE IODINE-125 PROSTATE BRACHYTHERAPY BOOST
Authors: Núria Bultó Boqué1, Alai Goñi Ramírez1, Belen de Paula Carranza1, Eva María Sáenz de Urturi Albisu1, María Pagola Divasson1, David Ignacio Ortiz de Urbina Ugarte1, Jesús Rosa Nieto1, Arantxa Ayete Andreu1, Melanie Erzilbengoa Izaguirre2, Vicent Pastor Sanchís2, Albert Bartrés Salido2, Noelia Suárez Álvarez2
1Onkologikoa UGC Onco-Hematologia Gipuzkoa, Radiation Oncology, San Sebastián, Spain; 2Onkologikoa UGC Onco-Hematologia Gipuzkoa , Medical Physics, San Sebastián, Spain
Show Affiliations
Hide Affiliations
Purpose or Objective
To describe the incidence, timing, and magnitude of the benign prostate-specific antigen (PSA) bounce after external beam radiotherapy (EBR) and low-dose-rate iodine-125 brachytherapy (LDR-BT) boost, its correlation with biochemical, clinical and/or dosimetric factors and the possible association between PSA bounce and Biochemical Failure (BF).
Material and Methods
From 2001 to 2015, 668 men with intermediate and high risk prostate cancer were treated with a combination of EBRT (46 Gy) and LDR-BT boost (108 Gy) with or without androgen deprivation therapy (ADT) in our institution. Of the 668 initial patients, 516 that had a PSA follow-up ≥ 60 months with PSA checked at least every 6 months were included for the analysis. An analysis of the subgroup of patients without ADT was performed. All patients received EBRT 46 Gy in 23 fractions of prostate irradiation and preplanned stranded-seed I-125 implant. PSA bounce was defined as an increase of ≥ 0.2 ng/ml with spontaneous return to prebounce level or lower without any intervention. BF was defined following the Phoenix criteria as PSA nadir + 2 ng/ml without any spontaneous decrease.
Results
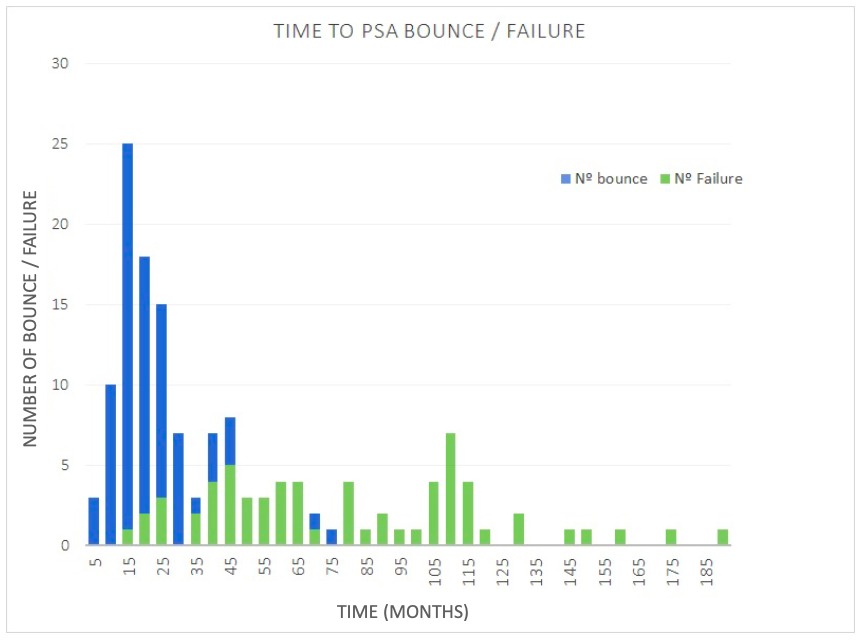
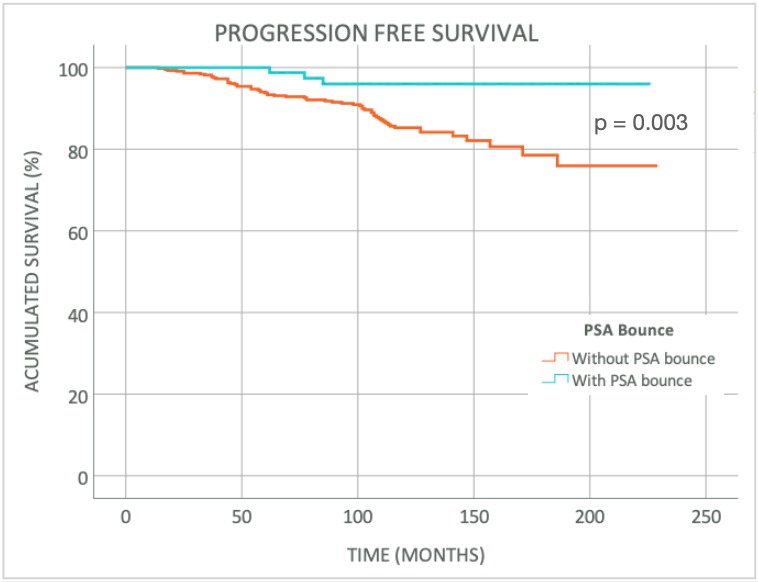
The median follow-up was 122 months (60-231 months). Stratified by risk, 289 (58.9%) patients had intermediate risk and 227 (41.1%), high risk of progression. Patients that received ADT were 286 (55.4%). PSA bounce was found in 81 (15.7%) men. Median prebounce PSA was 0.75 ng/ml (0.01-2.80 ng/ml) and median height of PSA bounce was 1.63 ng/ml (0.21-7.34 ng/ml). Median duration of PSA bounce was 17 months (6-47 months) and 40.7% of PSA bounce had a magnitude >2 ng/ml. BF was seen in 64 (12.4%) patients of whom 3 had a PSA bounce. Median time to PSA bounce was 17 months (3-73 months) whereas median time to BF was 73 months (14-186 months). Among 81 patients with a PSA bounce, 76 (93.8%) presented it before 40 months while, among 64 patients with BF, 61 (95.3%) presented it after 40 months. In the multivariate analysis, the younger age (<65 years) and the absence of ADT were predictor factors of PSA bounce. Progression Free Survival (PFS) at 5, 10 and 15 years were 99%, 96% and 96% in patients that present PSA bounce and 93%, 85% and 79% among patients without it (p 0.003). When we analyzed the subgroup of patients not treated with ADT (230 patients), 65 (28.3%) presented PSA bounce and the BF was seen in 32 (13.9%) men. Age (<65 years) was the only predictor factor in the multivariate analysis in this subgroup. PFS at 5, 10 and 15 years in this subgroup were 98%, 95% and 95% among the patients with PSA bounce and 91%, 82% and 74% among the ones without it (p 0.008).
Conclusion
Time to PSA bounce is commonly presented before 40 months, considerably shorter than time to BF. Benign PSA bounce is more frequent in younger men and those not treated with ADT. In the group of patients with PSA bounce, treated with or without ADT, PFS is improved with a lower incidence of BF among this group.